
As filed with the Securities and Exchange Commission on November 13, 2020
No. 333-249733
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 2
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Maravai LifeSciences Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 8731 | 85-2786970 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
10770 Wateridge Circle Suite 200
San Diego, California 92121
Telephone: (858) 546-0004
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Carl W. Hull
Chief Executive Officer
10770 Wateridge Circle Suite 200
San Diego, California 92121
Telephone: (858) 546-0004
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies of all communications, including communications sent to agent for service, should be sent to:
| Robert M. Hayward, P.C. Michael P. Keeley Kirkland & Ellis LLP |
Alan F. Denenberg Emily Roberts Menlo Park, California 94025 (650) 752-2000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☐
If this Form is filed to registered additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
|
| ||||||||
| Title of Each Class of Securities to be Registered |
Amount to be Registered(1) |
Proposed Maximum Offering Price Per Share(2) |
Proposed Maximum Offering Price(1)(2) |
Amount of Registration Fee(3) | ||||
| Class A Common Stock, par value $0.01 per share |
57,500,000 | $27.00 | $1,552,500,000 | $169,377.75 | ||||
|
| ||||||||
|
| ||||||||
| (1) | Includes the aggregate offering price of shares of common stock subject to the underwriters’ option to purchase additional shares. |
| (2) | Estimated solely for purposes of computing the amount of the registration fee pursuant to Rule 457(a) under the Securities Act of 1933. |
| (3) | The registrant previously paid a registration fee of $10,910 in connection with the initial filing of this Registration Statement. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. The prospectus is not an offer to sell these securities nor a solicitation of an offer to buy these securities in any jurisdiction where the offer and sale is not permitted.
PRELIMINARY PROSPECTUS (Subject to Completion)
Issued November 13, 2020
50,000,000 Shares

Class A Common Stock
This is the initial public offering of shares of Class A common stock of Maravai LifeSciences Holdings, Inc., par value $0.01 per share. Maravai LifeSciences Holdings, Inc. is offering 50,000,000 shares of its Class A common stock to be sold in the offering.
Prior to this offering, there has been no public market for the Class A common stock of Maravai LifeSciences Holdings, Inc. It is currently estimated that the initial public offering price per share will be between $24.00 and $27.00. Maravai LifeSciences Holdings, Inc. has been approved to have its Class A common stock listed on The Nasdaq Global Select Market under the symbol “MRVI.”
Maravai LifeSciences Holdings, Inc. has two authorized classes of common stock: Class A and Class B (together, the “common stock”). Holders of the Class A common stock and Class B common stock are each entitled to one vote per share. All holders of Class A common stock and Class B common stock will vote together as a single class except as otherwise required by applicable law. Holders of Class B common stock do not have any right to receive dividends or distributions upon the liquidation or winding up of Maravai LifeSciences Holdings, Inc.
Maravai LifeSciences Holdings, Inc. will use the net proceeds from this offering to purchase (i) newly-issued units (“LLC Units”) in Maravai Topco Holdings, LLC (“Topco LLC”) and (ii) outstanding LLC Units from Maravai Life Sciences Holdings, LLC (“MLSH 1”), the sole existing member of Topco LLC. The purchase price for the LLC Units will be equal to the initial public offering price of the shares of Class A common stock less the underwriting discounts and commissions referred to below. Topco LLC will use the net proceeds it receives from Maravai LifeSciences Holdings, Inc. in connection with this offering as described under “Use of Proceeds.” Upon completion of this offering, Maravai LifeSciences Holdings, Inc. will have 81,379,818 LLC Units representing a 32% economic interest in Topco LLC and, although Maravai LifeSciences Holdings, Inc. will initially have a minority economic interest in Topco LLC, it will be the sole managing member of Topco LLC and will operate and control its business. MLSH 1 will hold the remaining 176,458,692 LLC Units representing a 68% economic interest in Topco LLC. Each LLC Unit, together with one share of our Class B common stock, is, from time to time, exchangeable for one share of Class A common stock or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). Maravai LifeSciences Holdings, Inc. will be a holding company, and upon consummation of this offering and the application of the net proceeds therefrom, its sole asset will be LLC Units of Topco LLC. Immediately following this offering, the holders of Class A common stock will collectively own 100% of the economic interests in Maravai LifeSciences Holdings, Inc. and have 32% of the voting power of Maravai LifeSciences Holdings, Inc. MLSH 1, through ownership of our Class B common stock, will have the remaining 68% of the voting power of Maravai LifeSciences Holdings, Inc.
Maravai LifeSciences Holdings, Inc. is an “emerging growth company” as the term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, has elected to comply with certain reduced public company reporting requirements for this prospectus.
Investing in our Class A common stock involves risks. See “Risk Factors” beginning on page 26 to read about factors you should consider before buying shares of our Class A common stock.
Immediately after this offering, assuming an offering size as set forth above, funds controlled by our equity sponsor, GTCR, LLC (“GTCR”), will control approximately 81% of the combined voting power of our outstanding shares of Class A common stock and Class B common stock (or 78% if the underwriters’ option to purchase additional shares is exercised in full). As a result, we expect to be a “controlled company” within the meaning of the corporate governance standards of The Nasdaq Stock Market. See “Management—Corporate Governance—Controlled Company Status.”
PRICE $ A SHARE
| Per share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds, before expenses, to Maravai LifeSciences Holdings, Inc. |
$ | $ | ||||||
| (1) | See “Underwriters” for additional information regarding underwriting compensation. |
At our request, the underwriters have reserved up to 3% of the shares of Class A common stock offered by this prospectus for sale, at the initial public offering price, for sale to certain individuals through a directed share program, including our directors, certain employees and certain other individuals identified by management. See “Underwriters—Directed Share Program.”
The underwriters have the option to purchase up to an additional 7,500,000 shares of Class A common stock from us at the initial public offering price less the underwriting discounts and commissions for a period of 30 days after the date of this prospectus.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The underwriters expect to deliver shares of Class A common stock against payment in New York, New York on or about , 2020.
| MORGAN STANLEY | JEFFERIES | GOLDMAN SACHS & CO. LLC |
| BOFA SECURITIES | CREDIT SUISSE | UBS INVESTMENT BANK | ||
| BAIRD | WILLIAM BLAIR | STIFEL | KEYBANC CAPITAL MARKETS | |||
| ACADEMY SECURITIES | LOOP CAPITAL MARKETS | PENSERRA SECURITIES LLC | TIGRESS FINANCIAL PARTNERS | |||
, 2020.
Neither we nor any of the underwriters have authorized anyone to provide any information or make any representations other than that contained in this prospectus or in any free writing prospectus filed with the Securities and Exchange Commission (“SEC”). We and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell, and seeking offers to buy, shares of Class A common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the Class A common stock. Our business, financial condition, results of operations, and prospects may have changed since such date.
For investors outside of the United States, neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about, and to observe any restrictions relating to, this offering and the distribution of this prospectus outside of the United States.
Through and including , 2020 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
i
BASIS OF PRESENTATION
In connection with the consummation of this offering, we will effect certain organizational transactions. Unless otherwise stated or the context otherwise requires, all information in this prospectus reflects the consummation of the organizational transactions and this offering, which we refer to collectively as the “Organizational Transactions.” See “Organizational Structure” for a description of the Organizational Transactions and a diagram depicting our anticipated structure after giving effect to the Organizational Transactions, including this offering.
Unless we state otherwise or the context otherwise requires, the terms “we,” “us,” “our,” “our business,” “the Company” and “Maravai” refer to and similar references refer: (1) on or following the consummation of the Organizational Transactions, including this offering, to Maravai LifeSciences Holdings, Inc. and its consolidated subsidiaries, including Topco LLC, and (2) prior to the consummation of the Organizational Transactions, including this offering, to Topco LLC and its consolidated subsidiaries. The term “GTCR” or “our Sponsor” refers to GTCR, LLC, our equity sponsor, and the term “Topco LLC” refers to Maravai Topco Holdings, LLC.
We will be a holding company and the sole managing member of Topco LLC and, upon consummation of this offering and the application of net proceeds therefrom, our sole asset will be LLC Units of Topco LLC. Topco LLC is the predecessor of the issuer, Maravai LifeSciences Holdings, Inc., for financial reporting purposes. Maravai LifeSciences Holdings, Inc. will be the reporting entity following this offering.
Accordingly, this prospectus contains the historical financial statements of Topco LLC and its consolidated subsidiaries. The unaudited pro forma condensed consolidated financial data of Maravai LifeSciences Holdings, Inc. presented in this prospectus has been derived from the application of pro forma adjustments to the historical consolidated financial statements of Topco LLC and its subsidiaries included elsewhere in this prospectus. These pro forma adjustments give effect to the Organizational Transactions as described in “Organizational Structure,” including the consummation of this offering and other related transactions, as if all such transactions had occurred on January 1, 2019. See “Unaudited Pro Forma Condensed Consolidated Financial Information” for a complete description of the adjustments and assumptions underlying the unaudited pro forma consolidated financial data included in this prospectus.
MARKET AND INDUSTRY DATA
Unless otherwise indicated, information in this prospectus concerning economic conditions, our industry, our markets and our competitive position is based on a variety of sources, including information from independent industry analysts and publications, as well as our own estimates and research.
Our estimates are derived from publicly available information released by third-party sources, as well as data from our internal research, and are based on such data and our knowledge of our industry, which we believe to be reasonable. We have not had this information verified by any independent sources. The independent industry publications used in this prospectus were not prepared on our behalf. While we are not aware of any misstatements regarding any information presented in this prospectus, forecasts, assumptions, expectations, beliefs, estimates and projects involve risk and uncertainties and are subject to change based on various factors, including those described under the headings “Forward-Looking Statements” and “Risk Factors.”
ii
TRADEMARKS AND TRADENAMES
This prospectus includes our trademarks and service marks, “Maravai LifeSciences,” “TriLink BioTechnologies,” “Glen Research,” “Cygnus Technologies,” “Vector Laboratories,” “CleanCap®,” and “MockV™,” which are protected under applicable intellectual property laws and are the property of Maravai LifeSciences Holdings, Inc. or its subsidiaries. This prospectus also contains trademarks, service marks, trade names and copyrights of other companies which are the property of their respective owners. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names.
iii
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before investing in our Class A common stock. For a more complete understanding of us and this offering, you should read and carefully consider the entire prospectus, including the more detailed information set forth under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our consolidated financial statements and the related notes. Some of the statements in this prospectus are forward-looking statements. See “Forward-Looking Statements.” Unless otherwise stated, this prospectus assumes no exercise of the underwriters’ option to purchase additional shares.
OVERVIEW
We are a leading life sciences company providing critical products to enable the development of drug therapies, diagnostics, novel vaccines and support research on human diseases. Our more than 5,000 customers as of September 30, 2020 include the top 20 global biopharmaceutical companies ranked by research and development expenditures according to industry consultants, and many other emerging biopharmaceutical and life sciences research companies, as well as leading academic research institutions and in vitro diagnostics companies. Our products address the key phases of biopharmaceutical development and include complex nucleic acids for diagnostic and therapeutic applications, antibody-based products to detect impurities during the production of biopharmaceutical products, and products to detect the expression of proteins in tissues of various species.
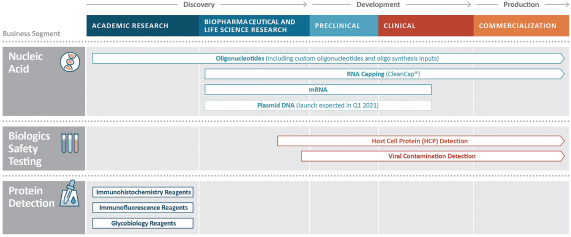
Our businesses principally serve high growth market segments in biopharmaceutical development and manufacturing. We estimate that the market segments we serve are growing at a weighted average blended rate of 20% per annum. In particular, the field of cell and gene therapy has emerged as one of the fastest growing treatment modalities to address a host of human conditions. There are more than 400 cell and gene therapies in development or launched and sales in this category are expected to grow more than tenfold by 2024, according to industry consultants and management estimates. Our portfolio offers key products for each stage of the cell and gene therapy development lifecycle. For example, our mRNA products are used in drug development to assist in the production of immune-activating antigens; our CleanCap® technology is used to stabilize mRNA; and we expect our upcoming plasmid DNA products will be used as templates for the production of our RNA products. We also provide biologics safety testing technology used to ensure the safety of the biological drug manufacturing process and drug products. We estimate that more than 64% of our revenue for the nine months ended September 30, 2020 was in support of vaccines and therapies in development, including biological drugs and cell and gene therapies.
1
Our proprietary capabilities and products underpin the value we aim to provide to our customers. Among other capabilities, we are experts in RNA and mRNA products, which are challenging and often unstable molecules requiring significant chemical modifications to ensure their stability and efficacy in our customers’ applications. Notably, according to research commissioned by us consisting of over 70 interviews with our current and former customers, our competitors and industry experts focused across our three business segments (the “Industry Analysis”), we believe CleanCap® is viewed as a leading solution to ensure the stability of mRNA. CleanCap® is a novel chemical approach to produce a cap analog, which, in addition to making mRNA more stable, aids in protein production and helps prevent an unwanted immune response to the mRNA. As of September 30, 2020, CleanCap® had been used by over 100 customers and had been incorporated into several development programs targeting immunization against the novel strain of coronavirus, SARS-CoV-2 (“COVID-19”). These programs included one phase II/III clinical program led by Pfizer in partnership with BioNTech, three phase I/II clinical programs led by Imperial College London, Fosun Pharma in partnership with BioNTech and CureVac and one pre-clinical program led by the University of Tokyo in partnership with Daiichi-Sankyo. In addition, CleanCap® is currently being used in three additional COVID-19 mRNA vaccine programs that are in earlier stages of development, led by Chula Vaccine Research Center in partnership with the University of Pennsylvania, eTheRNA Immunotherapies and Greenlight Biosciences. Given the early stage of these three programs, there can be no assurance they will continue to use CleanCap® through commercialization. We estimate our mRNA and CleanCap® products have also been incorporated in at least 33 therapeutic programs in development. Should one or more of these programs proceed to commercialization, we believe we will continue to supply our customers and our products will likely be incorporated in customer regulatory filings.
mRNA is at the core of our capabilities. We developed our expertise in mRNA with a belief in its potential as a therapeutic modality. With the COVID-19 pandemic, mRNA has shown its potential for more rapid vaccine design and manufacture when compared to traditional techniques involving culturing inactivated virus to elicit an immune response. According to the World Health Organization, there were 202 COVID-19 vaccine development programs as of November 3, 2020, with some of the lead candidates for approval in the RNA class. COVID-19 has helped highlight the potential advantage of mRNA as a treatment modality and directed significant resources to the developing base of knowledge about mRNA. We believe this knowledge will be directed at future vaccine programs as well as therapeutic agents for a host of human diseases. We are positioned to serve our biopharmaceutical customers in the fast-growing mRNA field across a range of clinical programs for a variety of diseases. Approximately 39% of our revenue was derived from products that support mRNA research for the nine months ended September 30, 2020.
Forming long-term partnerships with our customers is core to our strategy. We primarily serve our customers during the product development and process development phases. During product development, we collaborate with our customers to develop and synthesize nucleic acids, which in some cases comprise the active pharmaceutical ingredients (“APIs”) of our customers’ pre-commercial products. While we do not provide products that are themselves regulated as drugs or in vitro diagnostics, our customers frequently incorporate our products into their highly validated products and processes. For example, we provide oligonucleotides and antibody-based products used by in vitro diagnostic product manufacturers for their on-market products. Because of the extensive validation required for these products, these components are frequently purchased for the life of our customers’ products and we believe they are unlikely to be substituted. In addition, our analytical tools are used in the design and development of manufacturing processes and often will be used throughout the life cycle of our customers’ manufactured products. As a result, our customer relationships may span many years.
We believe we are a leader in providing critical products and solutions to life sciences customers worldwide to support all phases of biopharmaceutical development for innovative vaccines, therapies and diagnostics. The end markets we serve are growing rapidly, and we believe we will continue to benefit as biopharmaceutical companies increasingly look for partners like us with specialized and technical capabilities and products and services that span from research and development through commercialization. For the nine months ended September 30, 2020, we generated revenue of $185.7 million, representing 73% growth as compared to the nine months ended September 30, 2019. We generated net income of $64.3 million and Adjusted EBITDA of $104.8 million for the nine months ended September 30, 2020.
2
OUR PORTFOLIO AND CAPABILITIES
Our products address our customers’ needs for nucleic acid production, biologics safety testing and protein detection, and our operations are aligned to these three segments. For the nine months ended September 30, 2020, we sold more than 53% of our products and services to biopharmaceutical customers and our products serve high growth applications in vaccines, cell and gene therapies, biological drugs and molecular diagnostics.
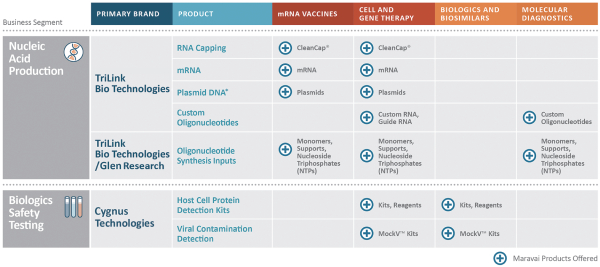
| * | Our plasmid DNA products are expected to launch in Q1 2021. |
Nucleic Acid Production (69% of Revenue for the Nine Months Ended September 30, 2020)
We are a global provider of highly modified, complex nucleic acids and related products. We have recognized expertise in complex chemistries and products provided under exacting quality standards. Our core offerings include mRNA, long and short oligonucleotides, our proprietary CleanCap® capping technology and oligonucleotide building blocks. We offer a suite of CleanCap® analogs that are specifically made for therapeutics and vaccines. Based on the Industry Analysis, we believe our cap analogs are critical features of several mRNA vaccines in development. Our offerings address key customer needs for critical components, from research-grade to good manufacturing practices (“GMP”) grade materials. We market our nucleic acid products under the TriLink BioTechnologies and Glen Research brands.
Biologics Safety Testing (22% of Revenue for the Nine Months Ended September 30, 2020)
We provide products and services under the Cygnus Technologies brand that ensure the purity of our customers’ biopharmaceutical products, including biological drugs. For over 20 years, the Cygnus Technologies brand has been associated with products and services that enable the detection of impurities and contaminants present in bioproduction. Our biologics safety testing products are used during development and scale-up, during the regulatory approval process and throughout commercialization. We are recognized globally for the detection of host cell proteins (“HCPs”) and process-related impurities during bioproduction.
Protein Detection (9% of Revenue for the Nine Months Ended September 30, 2020)
We believe that we are a leader in labeling and detection reagents for immunohistochemistry, immunofluorescence and glycobiology, principally in research settings, with Vector Laboratories, the brand under which we market our protein detection products, having been cited over 350,000 times in scientific
3
publications. Our products are used to detect the expression of proteins in tissue, which may indicate an ongoing disease process, with the use of antibody-based detection systems. We also manufacture lectins, proteins that preferentially bind to carbohydrates and which are used, for example, in the study of glycosylation, the process by which carbohydrates attach to proteins and lipids. Glycosylation is critical in a range of biological processes, including cell-to-cell adhesion, the performance of glycoprotein-based drugs and cancer. In addition, we manufacture bioconjugation reagents to allow rapid and quantifiable conjugation of all classes of biomolecules.
OUR COMPETITIVE STRENGTHS
We believe we are a leader in providing nucleic acid products and biologics safety testing products to biopharmaceutical customers worldwide. Our success is built on our ability to provide proprietary technologies and products under exacting quality standards to reliably serve our customers’ needs for critical raw materials.
Leading Supplier of Critical Solutions for Life Sciences from Discovery to Commercialization
We seek to be an important component of our customers’ supply chain by providing inputs that are central to the performance of their products and processes throughout the product lifecycle. By collaborating with customers early in the development phase, our products frequently follow our customers’ development path to commercialization and are likely to be incorporated as raw materials in their on-market products and processes. Our decades-long experience and track record, coupled with our ongoing investment in facilities and quality systems, allow our customers to rely on us for their critical products. Our approach is to be a trusted partner throughout the life cycle of our customers’ products.
Innovation, Proprietary Technologies and Knowhow Underpin Our Portfolio
Our expertise in complex chemistries leads customers to seek our collaboration in designing complex products that meet high performance expectations. Based on the responses to the Industry Analysis, we believe the solutions we provide, in many cases, cannot be provided effectively by our competitors. In certain cases, like our CleanCap® technology, our knowhow is backed by intellectual property. In other cases, such as our HCP products, our antibodies are proprietary and therefore can only be supplied by us. We believe the proprietary nature of our knowhow and products solidifies our long-term customer relationships.
Products with Outstanding Performance
We believe our products stand out when compared to our competitors because they present innovative solutions to customer needs, as indicated by the responses to the Industry Analysis, while providing reliable performance and quality. CleanCap®, for example, offers advantages over competing technologies in yield, stability and safety. Our oligonucleotides address complex chemistry challenges, which few competitors can address. The results of the Industry Analysis indicate that our HCP ELISAs have defined the market for impurity detection and we believe they have become a de facto standard in biologics safety testing. Our protein detection assays have been recognized for their performance for over 40 years.
Trusted Brands
Our TriLink BioTechnologies, Glen Research, Cygnus Technologies and Vector Laboratories product brands are well known in their respective markets for consistent quality and performance. This brand recognition has been earned over decades. Our manufacturing processes, quality standards, technical support and high-touch customer service ensure that we maintain the reputation of our brands.
State-of-the-Art Manufacturing Facilities
Our biopharmaceutical customers manufacture their products to meet stringent quality standards and expect their critical suppliers to meet their exacting requirements. Our customers further expect that we have the
4
production capacity to meet their needs. As of September 30, 2020, we estimate that $70.0 million has been invested in our flagship San Diego, California facility and manufacturing suites to produce materials under GMP conditions, along with the necessary quality systems, to meet requirements specified by our customers. We similarly invest in our other sites to ensure we meet our customers’ expectations. We believe that the capacity to manufacture to stringent biopharmaceutical standards is constrained in the industry and our ability to meet this demand sets us apart from our competition.
Experienced Leaders and Talented Workforce
Our management includes experienced leaders with demonstrated records of success at Maravai and other highly regarded industry participants. In addition, as of September 30, 2020, approximately 17% of our workforce have advanced degrees and all receive rigorous on the job training. We believe the quality of our personnel is critical to ensuring the collaborative, long-standing relationships we maintain with many of our customers.
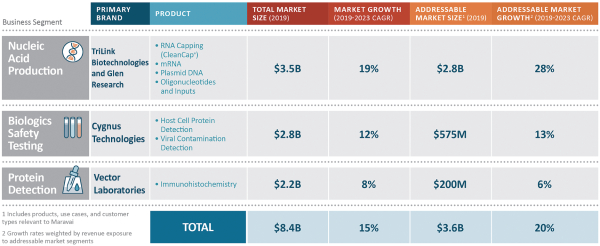
OUR MARKETS
We participate in three distinct market segments: nucleic acid production, biologics safety testing and protein detection, which together represented approximately $8.4 billion in annual spending in 2019 and which are expected to grow at a 15% compound annual growth rate (“CAGR”) through 2023 according to industry consultants and management estimates. Of that combined market, we estimate our addressable portion represents approximately $3.6 billion. Our addressable segments, adjusted for the mix of products we offer, are expected to grow at a weighted average blended rate of 20% per annum through 2023. We benefit from favorable industry dynamics in our broader market segments and specific growth drivers in our addressable market segments.
The biopharma space remains well-funded, as demonstrated by the substantial amount of capital that has been raised in recent years. In addition, the level of capital markets activity in this space over recent years underscores the rapid pace of biopharmaceutical innovation and increasing cost of drug development. According to Dealogic, the amount raised in initial public offerings and follow-on offerings by biotechnology companies in the U.S. public capital markets between 2015 and 2020 was more than $195.0 billion. This includes over $45.0 billion raised by companies across more than 228 initial public offerings, reflecting an average of 40 initial public offerings of biotechnology companies per year from 2015 to the six-month period ended June 30, 2020.
Biopharmaceutical customers are increasingly relying on outside parties to provide important inputs and services for their clinical research and manufacturing, a development driving growth for suppliers with unique capabilities and the ability to manufacture at an appropriate scale to support customer programs. We believe that
5
suppliers like ourselves, with this rare combination of capabilities, proprietary products and the required investment in manufacturing and quality systems, are benefiting from rapid growth as biopharmaceutical customers seek to partner with a small number of trusted suppliers.
In addition to the continued trend toward outsourcing, several market developments are driving increased growth, above the broader market growth rates, in our addressable market segments, including:
| • | Pivot toward mRNA vaccines, driven in part by COVID-19. mRNA vaccine pre-clinical programs grew approximately 38% in 2019, before the COVID-19 pandemic. That rate is expected to increase to approximately 63% in 2020. The increased growth is being driven, in part, by 26 COVID-19 vaccine programs using mRNA as of November 3, 2020 according to the World Health Organization. Five of the 26, including one phase II/III clinical program led by Pfizer in partnership with BioNTech, three phase I/II clinical programs led by Imperial College London, Fosun Pharma in partnership with BioNTech and CureVac and one pre-clinical program led by the University of Tokyo in partnership with Daiichi-Sankyo, involve our CleanCap® products and up to three more in earlier stages of development, led by Chula Vaccine Research Center in partnership with the University of Pennsylvania, eTheRNA Immunotherapies and Greenlight Biosciences are currently using our CleanCap® products. Given the early stage of these three programs, there can be no assurance they will continue to use CleanCap® through commercialization. mRNA vaccine technology is gaining prominence as a result of its faster development time, lower manufacturing costs and improved safety because of the lower risk of unwanted immune responses. RNA expertise is highly specialized and customers seek partners to provide these complex products. A small number of providers, like ourselves, can provide this RNA capability. |
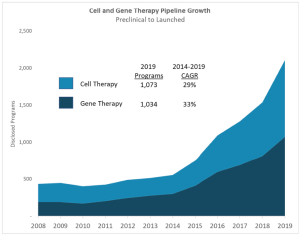
| • | Rapid growth in development of cell and gene therapies. Sales of cell and gene therapy drugs are expected to grow from $1 billion in 2019 to $25 billion by 2024, according to industry consultants. We support the development of these therapies with products used in gene editing and cell therapy research, and we are well positioned to supply materials for gene therapy with our launch of DNA plasmid products, which we expect in the first quarter of 2021. |
|
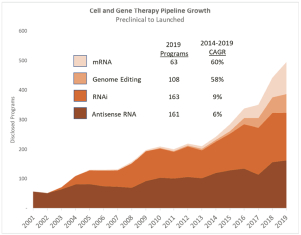
|
| • | Large and growing pipeline of protein-based therapeutics. In addition to cell and gene therapies, an increase in protein-based therapies is driving the need for impurity testing during process development and manufacturing. |
6
| Protein Therapy Pipeline Growth CAGR = 9%
|
Protein Therapy Pipeline Mix CAGR = 9%
|
Source: Industry consultants. Included proteins are biosimilars, antibodies, recombinant proteins, hormonal products and coagulation factor
| • | Rise in molecular diagnostics, driven by COVID-19: The market for molecular diagnostics is growing dramatically because of demand for new tests related to COVID-19. This growth is driving demand for our products, particularly oligonucleotides and related inputs. |
| • | COVID-19 providing both short-term and expected long-term growth: Several of our product categories are experiencing accelerated growth in 2020, notably our CleanCap® and oligonucleotide products. We expect the impact of COVID-19 on our growth to sustain in the longer term as the entire mRNA category benefits from lessons learned during the COVID-19 pandemic. We expect research in other therapeutic categories to experience increased growth as research conducted for COVID-19 diffuses more broadly into other vaccines and therapies. |
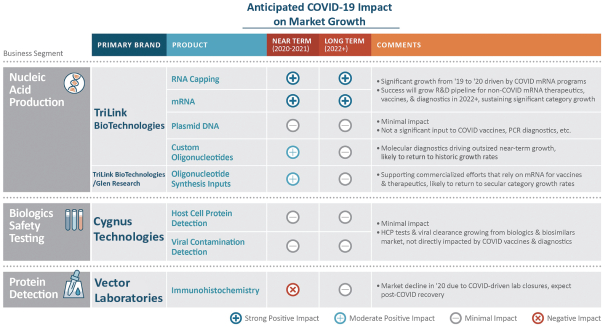
| * | Our plasmid DNA products are expected to launch in Q1 2021. |
7
OUR STRATEGY
Our customers strive to improve human health. Our goal is to provide them with products and services to accelerate their development efforts, from basic research through clinical trials and ultimately to commercialization for drugs, diagnostics and vaccines.
Supporting Biopharma Customers from Product Development through Commercialization
Our customers include both emerging and established biopharmaceutical leaders developing novel therapies, diagnostics and vaccines. Emerging biopharmaceutical customers frequently seek the support we can offer in our state-of-the-art facilities under our stringent quality standards, with the capabilities that result from the capital and process investments we have made over the last several years. Although our products are exempt from current GMP regulations, we are capable of manufacturing reagents from research-grade to GMP-grade, which often exceeds the in-house capabilities of our pre-commercial customers. See “Business—Government Regulation.” The results of the Industry Analysis indicate that our emerging and established customers also seek us out for our leading capabilities in nucleic acid chemistries, especially in highly modified nucleic acids and mRNA, and process control assays. We further support our customers as they transition from product development to commercialization by providing critical raw materials for their drugs.
Developing Proprietary Technologies that Deepen Our Relationships with Our Customers
We are experts in nucleic acids and our scientists aim to develop proprietary enabling technologies that become integral to our customers’ products. For example, CleanCap®, our proprietary chemical capping technology, has demonstrated its advantages in terms of the stability of the associated mRNA and its efficiency in protein production when compared to traditional capping technologies. This efficiency has led biopharmaceutical customers to employ CleanCap® in their vaccine and therapeutic programs. As those products proceed through development into commercialization, we believe CleanCap® will be a critical input in on-market vaccines and therapeutics, with over 100 customers having used CleanCap® as of September 30, 2020 and five COVID-19 vaccine programs incorporating CleanCap® as of September 30, 2020, including one phase II/III clinical program led by Pfizer in partnership with BioNTech, three phase I/II clinical programs led by Imperial College London, Fosun Pharma in partnership with BioNTech and CureVac and one pre-clinical program led by the University of Tokyo in partnership with Daiichi-Sankyo. We expect to supply our customers throughout their products’ life cycle.
Forming Long-Term Partnerships for Critical Biopharmaceutical Components and Process Tests
Our products are frequently incorporated into regulated and highly validated therapeutic and diagnostic products and processes. Our biopharmaceutical customers expect us to provide them with consistent, high quality products that meet narrow specifications, and that we ensure their supply chain for such products for the length of their programs. In many cases, we may be the sole source of the products we provide. Our emphasis on partnership generally leads to long-term relationships with our customers.
Focusing Our Efforts on High Growth End Markets
While biopharmaceutical research and in vitro diagnostics markets are experiencing strong growth, we target the highest growth segments within those markets. Our product portfolio is well positioned to serve the biologics, cell and gene therapy and mRNA vaccine and therapeutic end markets, which are currently experiencing above-market growth. By investing in technologies at the forefront of biopharmaceutical and in vitro diagnostics, we aim to remain focused on the highest-growth applications.
8
Opportunistically Acquiring Leading Life Sciences Businesses and Supporting Their Continued Development
We built our business by acquiring established and emerging companies with strong scientific foundations in our target markets and investing in their systems, processes and people to accelerate their growth and expand their technologies. Going forward, we may opportunistically pursue strategic acquisitions that meet, or could meet after being acquired and expanded, the following criteria:
| • | address our core target markets; |
| • | have a demonstrated adherence to high quality standards; |
| • | be leaders in their market niches; |
| • | have differentiated or proprietary products and processes that provide clear value to our biopharmaceutical and other customers; and |
| • | have a track record of attractive rates of growth and compelling returns on invested capital. |
Our acquisition strategy is to invest significantly in our acquired businesses. We strive to rapidly integrate their information and financial systems, seek opportunities to invest in their facilities and personnel and augment their commercial capabilities through a combination of sales and marketing resources dedicated to each business, supported by our global marketing infrastructure. We will continue to seek a balance between driving growth organically and through opportunistic acquisitions.
RISKS ASSOCIATED WITH OUR BUSINESS
There are a number of risks related to our business, this offering and our Class A common stock that you should consider before you decide to participate in this offering. You should carefully consider all the information presented in the section entitled “Risk Factors” in this prospectus. Some of the principal risks related to our business include the following:
| • | our history of losses, the risk that we may continue to incur losses in the future and our ability to generate sufficient revenue to achieve or maintain profitability; |
| • | the fluctuation of our operating results, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide; |
| • | our dependence on a limited number of customers for a high percentage of our revenue; |
| • | the use of certain of our products in the production of vaccines and therapies that represent relatively new and still-developing modes of treatment, which may experience unforeseen adverse events, negative clinical outcomes or increased regulatory scrutiny; |
| • | the impact of COVID-19 and any pandemic, epidemic or outbreak of infectious disease; |
| • | changes in economic conditions; |
| • | our dependence on customers’ spending on and demand for outsourced nucleic acid production, biologics safety testing and protein detection research products and services; |
| • | competition with life science, pharmaceutical and biotechnology companies who are substantially larger than we are and potentially capable of developing new approaches that could make our products, services and technologies obsolete; |
| • | the ability of our products and services to perform as expected and the reliability of the technology on which our products and services are based; |
9
| • | the complexity of our products and the fact that they are subject to quality control requirements; |
| • | our reliance on a limited number of suppliers or, in some cases, sole suppliers, for some of our raw materials and our inability to find replacements or immediately transition to alternative suppliers; |
| • | our dependence on a stable and adequate supply of quality raw materials from our suppliers, and the risk of adverse impacts from price increases or interruptions of such supply; |
| • | disruptions at our sites; |
| • | our ability to manufacture in specific quantities; |
| • | natural disasters, geopolitical unrest, war, terrorism, public health issues such as COVID-19 or other catastrophic events that could disrupt the supply, delivery or demand of products and services; |
| • | our ability to secure additional financing for future strategic transactions; |
| • | our reliance on third-party package deliver services and adverse impacts arising from significant disruptions of these services, damages or losses sustained during shipping or significant increases in prices; |
| • | our ability to continue to hire and retain skilled personnel; |
| • | our ability to successfully identify and implement distribution arrangements and marketing alliances; |
| • | the market acceptance of our life science reagents; |
| • | the market receptivity to our new products and services upon their introduction; |
| • | our ability to obtain, maintain and enforce intellectual property protection for our current and future products; and |
| • | the other factors set forth under “Risk Factors.” |
These and other risks are more fully described in the section entitled “Risk Factors” in this prospectus. If any of these risks actually occurs, our business, financial condition, results of operations, cash flows and prospects could be materially and adversely affected. As a result, you could lose all or part of your investment in our Class A common stock.
OUR SPONSOR
We have a valuable relationship with our equity sponsor, GTCR. In connection with this offering, we will enter into a director nomination agreement (the “Director Nomination Agreement”) with GTCR that provides GTCR the right to designate nominees to our board of directors (the “Board”), subject to certain conditions. See “Certain Relationships and Related Party Transactions—Director Nomination Agreement” for more details with respect to the Director Nomination Agreement.
Founded in 1980, GTCR is a leading growth-oriented private equity firm focused on investing in growth companies in the Healthcare, Financial Services & Technology, Technology, Media & Telecommunications and Growth Business Services industries. The Chicago-based firm pioneered The Leaders Strategy™—finding and partnering with management leaders in core domains to identify, acquire and build market-leading companies through transformational acquisitions and organic growth. Maravai is an example of the Leaders Strategy™, whereby GTCR is partnering with Carl Hull and Eric Tardif to build and grow a leading life sciences platform. Since its inception, GTCR has invested more than $18.0 billion in over 200 companies.
10
GENERAL CORPORATE INFORMATION
Our principal executive offices are located at 10770 Wateridge Circle Suite 200, San Diego, California, 92121. Our telephone number is (858) 546-0004. Our website address is www.maravai.com. The information contained on, or that can be accessed through, our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our Class A common stock. We are a holding company and all of our business operations are conducted through, and substantially all of our assets are held by, our subsidiaries.
IMPLICATIONS OF BEING AN EMERGING GROWTH COMPANY
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year following the fifth anniversary of the completion of this offering, (2) the last day of the fiscal year in which we have total annual gross revenue of at least $1.07 billion, (3) the date on which we are deemed to be a large accelerated filer (this means the market value of common that is held by non-affiliates exceeds $700.0 million as of the end of the second quarter of that fiscal year), or (4) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| • | not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (the “Sarbanes-Oxley Act”); |
| • | only required to present two years of audited financial statements, plus unaudited condensed financial statements for any interim period, and related management’s discussion and analysis of financial condition and results of operations; |
| • | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
| • | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We have elected to take advantage of certain of the reduced disclosure obligations regarding financial statements and executive compensation in this prospectus and expect to elect to take advantage of other reduced burdens in future filings. As a result, the information that we provide to our shareholders may be different than you might receive from other public reporting companies in which you hold equity interests.
Under the JOBS Act emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to avail ourselves of the extended transition period for complying with new or revised financial accounting standards. As a result of the accounting standards election, we will not be subject to the same implementation timing for new or revised accounting standards as other public companies that are not emerging growth companies, which may make comparison of our financials to those of other public companies more difficult.
OWNERSHIP AND ORGANIZATIONAL STRUCTURE
Maravai LifeSciences Holdings, Inc. is a Delaware corporation formed to serve as a holding company that will hold an interest in Topco LLC. Maravai LifeSciences Holdings, Inc. has not engaged in any business or other activities other than in connection with its formation and this offering. Upon consummation of this offering
11
and the application of the proceeds therefrom, we will be a holding company, our sole asset will be an equity interest in Topco LLC and we will operate and control all of the business and affairs and consolidate the financial results of Topco LLC. See “Organizational Structure” for a complete description of the Organizational Transactions.
In connection with the Organizational Transactions:
| • | We will amend and restate Topco LLC’s existing operating agreement (the “LLC Operating Agreement”) to, among other things, (i) modify Topco LLC’s capital structure by replacing the membership interests currently held by Topco LLC’s existing owners (beneficially owned through MLSH 1) with a new class of LLC Units held initially by MLSH 1 and (ii) appoint Maravai LifeSciences Holdings, Inc. as the sole managing member of Topco LLC. See “Organizational Structure—Amended and Restated Operating Agreement of Topco LLC. |
| • | Certain of the entities (the “Blocker Entities”) through which GTCR and other existing members of MLSH 1 hold their ownership interests in MLSH 1 will form Maravai Life Sciences Holdings 2, LLC (“MLSH 2”) and engage in a series of transactions (the “Blocker Mergers”) that will result in each of the Blocker Entities merging with and into Maravai LifeSciences Holdings, Inc., with Maravai LifeSciences Holdings, Inc. remaining as the surviving corporation. As a result of such transactions, (i) the former equityholders of the Blocker Entities will become members of MLSH 2 and (ii) MLSH 2 will exchange all of the equity interests in the Blocker Entities for (x) shares of Class A common stock and (y) the right to receive payments pursuant to the Tax Receivable Agreement. |
| • | We will enter into an exchange agreement (the “Exchange Agreement”) with MLSH 1 pursuant to which MLSH 1 will be entitled to exchange LLC Units, together with an equal number of shares of Class B common stock, for shares of Class A common stock on a one-for-one basis or, at our election, for cash, from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). See “Organizational Structure—Exchange Agreement.” |
| • | We will enter into a tax receivable agreement (the “Tax Receivable Agreement”) with MLSH 1 and MLSH 2 that will provide for the payment by Maravai LifeSciences Holdings, Inc. to MLSH 1 and MLSH 2, collectively, of 85% of the amount of cash savings, if any, in U.S. federal, state and local income taxes (computed using simplifying assumptions to address the impact of state and local taxes) we actually realize (or, under certain circumstances are deemed to realize in the case of an early termination payment by us, a change in control or a material breach by us of our obligations under the Tax Receivable Agreement, as discussed below) as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we are required to make under the Tax Receivable Agreement. See “Organizational Structure—Tax Receivable Agreement.” |
| • | We estimate that the net proceeds to us from the sale of our Class A common stock in this offering, after deducting underwriting discounts and commissions and estimated expenses payable by us, will be approximately $1,184.5 million ($1,365.2 million if the underwriters exercise their option to purchase additional shares in full), based on an assumed initial public offering price of $25.50 per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus). We intend to use such net proceeds as follows: |
| • | $94.5 million to acquire 3,921,569 newly-issued LLC Units in Topco LLC and $942.7 million to acquire 39,121,430 outstanding LLC Units from MLSH 1 (or $1,096.2 million to acquire 45,489,067 outstanding LLC Units if the underwriters exercise their option to purchase additional |
12
| shares in full), in each case at a purchase price per LLC Unit equal to the initial offering price per share of Class A common stock in this offering, less underwriting discounts and commissions; and |
| • | $167.6 million to pay MLSH 2 as consideration for the Blocker Mergers and, if the underwriters exercise their option to purchase additional shares in full, $27.3 million to acquire outstanding shares of Class A common stock from MLSH 2 at a purchase price per share equal to the initial public offering price per share of Class A common stock in this offering, less underwriting discounts and commissions. |
In turn, Topco LLC intends to apply the balance of the net proceeds it receives from us (including any additional proceeds it may receive from us if the underwriters exercise their option to purchase additional shares) to (i) repay $50.0 million of our outstanding indebtedness under the New Credit Agreement, under which $600.0 million was outstanding and which had an interest rate of 5.25% as of October 31, 2020, (ii) pay expenses incurred in connection with this offering and the Organizational Transactions and (iii) for general corporate purposes. See “Use of Proceeds.”
The diagram below depicts our historical organizational structure prior to the completion of the Organizational Transactions. This diagram is provided for illustrative purposes only and does not purport to represent all legal entities owned or controlled by us, or owning a beneficial interest in us.
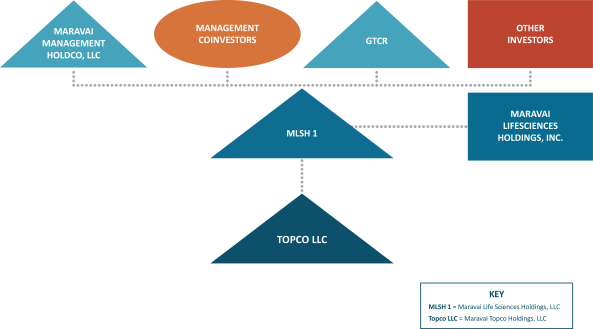
13
The diagram below depicts our expected organizational structure immediately following completion of the Organizational Transactions. This diagram is provided for illustrative purposes only and does not purport to represent all legal entities owned or controlled by us, or owning a beneficial interest in us.
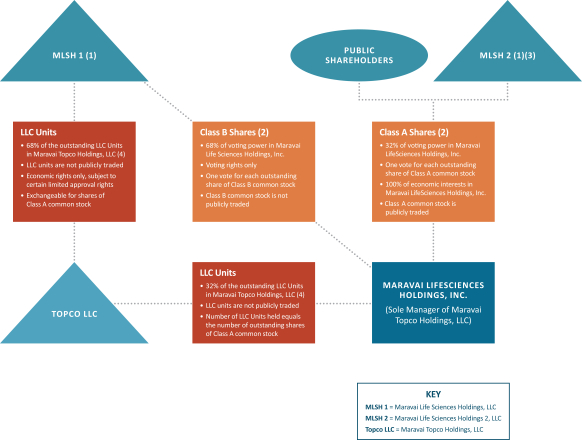
| (1) | Upon completion of this offering, GTCR will control the voting power in Maravai LifeSciences Holdings, Inc. as follows: (i) approximately 68% (or approximately 66% if the underwriters exercise their option to purchase additional shares in full) through its control of MLSH 1 and (ii) approximately 12% through its control of MLSH 2. See “Principal Shareholders” for additional information about MLSH 1 and MLSH 2. |
| (2) | Shares of Class A common stock and Class B common stock will vote as a single class. Each outstanding share of Class A common stock and Class B Common stock will be entitled to one vote on all matters to be voted on by shareholders generally. The Class B common stock does not have any right to receive dividends or distributions upon the liquidation or winding up of Maravai LifeSciences Holdings, Inc. In accordance with the Exchange Agreement to be entered into in connection with the Organizational Transactions, MLSH 1 will be entitled to exchange LLC Units, together with an equal number of shares of Class B common stock, for shares of Class A common stock determined in accordance with the Exchange Agreement or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). |
| (3) | Upon completion of this offering, we expect to award options to purchase an aggregate of 1,584,400 shares of Class A common stock with an exercise price set at the initial public offering price issued pursuant to the 2020 Omnibus Incentive Plan (the “2020 Plan”). |
| (4) | Assumes no exercise of the underwriters’ option to purchase additional shares. If the underwriters exercise their option to purchase additional shares in full, (i) the holders of Class A common stock will have 34% of |
14
| the voting power in Maravai LifeSciences Holdings, Inc., (ii) MLSH 1, through ownership of the Class B common stock, will have 66% of the voting power of Maravai LifeSciences Holdings, Inc., (iii) MLSH 1 will own 66% of the outstanding LLC Units in Topco LLC and (iv) Maravai LifeSciences Holdings, Inc. will own 34% of the outstanding LLC Units in Topco LLC. |
Our corporate structure following the offering, as described above, is commonly referred to as an “Up-C” structure, which is commonly used by partnerships and limited liability companies when they undertake an initial public offering of their business. Our Up-C structure together with the Tax Receivable Agreement will allow the existing owners of Topco LLC to continue to realize tax benefits associated with owning interests in an entity that is treated as a partnership, or “passthrough” entity, for income tax purposes following the offering. One of these benefits is that future taxable income of the Topco LLC that is allocated to such owners will be taxed on a flow-through basis and therefore will not be subject to corporate taxes at the entity level. Additionally, because the LLC Units that the existing owners will continue to hold are exchangeable for shares of our Class A common stock or, at our option, for cash, from Topco LLC, the Up-C structure also provides the existing owners of Topco LLC potential liquidity that holders of non-publicly traded limited liability companies are not typically afforded. See “Organizational Structure” and “Description of Capital Stock.”
Following this offering, MLSH 1 will hold a number of shares of our Class B common stock equal to the number of LLC Units it owns. Holders of our Class A common stock and Class B common stock will each be entitled to one vote per share on all matters on which shareholders are entitled to vote.
Maravai LifeSciences Holdings, Inc. will also hold LLC Units, and therefore receive benefits on account of its ownership in an entity treated as a partnership, or “passthrough” entity, for income tax purposes. As Maravai LifeSciences Holdings, Inc. purchases LLC Units from MLSH 1 under the mechanism described above, it will obtain a step-up in tax basis in its share of the assets of Topco LLC and its flow-through subsidiaries. This step-up in tax basis will provide Maravai LifeSciences Holdings, Inc. with certain tax benefits, such as future depreciation and amortization deductions that can reduce the taxable income allocable to Maravai LifeSciences Holdings, Inc. Pursuant to the Tax Receivable Agreement, Maravai LifeSciences Holdings, Inc. will agree to pay MLSH 1 and MLSH 2, collectively, 85% of the value of these tax benefits; however, the remaining 15% of such benefits will be available to Maravai LifeSciences Holdings, Inc. Due to the uncertainty of various factors, we cannot precisely quantify the likely tax benefits we will realize as a result of LLC Unit exchanges and the resulting amounts we are likely to pay out to LLC Unitholders pursuant to the Tax Receivable Agreement; however, we estimate that such payments may be substantial. See “Organizational Structure—Tax Receivable Agreement.”
Generally, Maravai LifeSciences Holdings, Inc. will receive a pro rata share of any distributions (including tax distributions) made by Topco LLC to its members. Tax distributions will be calculated without regard to any applicable basis adjustment under Section 743(b) of the Internal Revenue Code (the “Code”) and will be based upon an assumed tax rate, which, under certain circumstances, may cause Topco LLC to make tax distributions that, in the aggregate, exceed the amount of taxes that Topco LLC would have paid if it were a similarly situated corporate taxpayer. Funds used by Topco LLC to satisfy its tax distribution obligations will not be available for reinvestment in our business. See “Risk Factors—Risks Related to Our Organizational Structure.”
As a result of the Organizational Transactions:
| • | the investors in this offering will collectively own 50,000,000 shares of our Class A common stock and we will hold 81,379,818 LLC Units; |
| • | MLSH 1 will own 176,458,692 LLC Units and 176,458,692 shares of Class B common stock; |
| • | our Class A common stock will collectively represent approximately 32% of the voting power in us, with shares of Class A common stock held by the public representing approximately 19% of the voting power in us; and |
| • | our Class B common stock will collectively represent approximately 68% of the voting power in us. |
15
THE OFFERING
| Issuer |
Maravai LifeSciences Holdings, Inc. | |
| Class A common stock offered by us |
50,000,000 shares. | |
| Underwriters’ option to purchase additional shares of Class A common stock |
7,500,000 shares. | |
| Class A common stock to be outstanding immediately after this offering |
81,379,818 shares (or 87,747,455 shares if the underwriters’ option is exercised in full). If all outstanding LLC Units held by MLSH 1 were exchanged for newly-issued shares of Class A common stock on a one-for-one basis, 257,838,510 shares of Class A common stock would be outstanding. | |
| Class B common stock to be outstanding immediately after this offering |
176,458,692 shares. Immediately after this offering, MLSH 1 will own 100% of the outstanding shares of our Class B common stock. | |
| Ratio of shares of Class A common stock to LLC Units |
Our amended and restated certificate of incorporation and the amended and restated operating agreement of Topco LLC will require that we and Topco LLC at all times maintain a one-to-one ratio between the number of shares of Class A common stock issued by us and the number of LLC Units owned by us (subject to certain exceptions for treasury shares and shares underlying certain convertible or exchangeable securities). | |
| Voting |
Each share of our Class A common stock entitles its holder to one vote on all matters to be voted on by shareholders generally.
Each share of our Class B common stock entitles its holder to one vote on all matters to be voted on by shareholders generally.
After this offering, MLSH 1 will hold a number of shares of Class B common stock equal to the number of LLC Units it owns. See “Description of Capital Stock—Class B Common Stock.”
Holders of our Class A common stock and Class B common stock vote together as a single class on all matters presented to our shareholders for their vote or approval, except as otherwise required by applicable law. | |
16
| Voting power held by holders of Class A common stock |
32% (or 100% if all outstanding LLC Units were exchanged for newly-issued shares of Class A common stock on a one-for-one basis). | |
| Voting power held by holders of Class B common stock |
68% (or 0% if all outstanding LLC Units were exchanged for newly-issued shares of Class A common stock on a one-for-one basis). | |
| Use of proceeds |
We estimate, based upon an assumed initial public offering price of $25.50 per share (which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), we will receive net proceeds from this offering of approximately $1,184.5 million (or $1,365.2 million if the underwriters exercise their option to purchase additional shares in full), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds as follows:
• $94.5 million to acquire 3,921,569 newly-issued LLC Units in Topco LLC and $942.7 million to acquire 39,121,430 outstanding LLC Units (or $1,160.0 million to acquire 45,489,067 LLC Units if the underwriters exercise their option to purchase additional shares in full) from MLSH 1, in each case at a purchase price per LLC Unit equal to the initial public offering price per share of Class A common stock in this offering, less underwriting discounts and commissions; and
• $167.6 million to pay MLSH 2 as consideration for the Blocker Mergers and, if the underwriters exercise their option to purchase additional shares in full, $27.3 million to acquire outstanding shares of Class A common stock from MLSH 2 at a purchase price per share equal to the initial public offering price per share of Class A common stock in this offering, less underwriting discounts and commissions. | |
| In turn, Topco LLC intends to:
• use $50.0 million to repay a portion of the amount outstanding under our New Credit Agreement, under which $600.0 million was outstanding and which had an interest rate of 5.25% as of October 31, 2020; and | ||
17
|
• apply the balance of the net proceeds it receives from us (including any additional proceeds it may receive from us if the underwriters exercise their option to purchase additional shares) to pay expenses incurred in connection with this offering and the Organizational Transactions and for general corporate purposes.
See “Use of Proceeds” and “Organizational Structure.” | ||
| Controlled company |
After this offering, assuming an offering size as set forth in this section, GTCR will control approximately 81% of the voting power (or 78% if the underwriters’ option to purchase additional shares is exercised in full) in us. As a result, we expect to be a controlled company within the meaning of the corporate governance standards of The Nasdaq Stock Market (“NASDAQ”). See “Management—Corporate Governance—Controlled Company Status.” | |
| Directed share program |
At our request, the underwriters have reserved up to 1,725,000 shares of our Class A common stock, or 3% of the shares of our Class A common stock to be offered by this prospectus for sale, at the initial public offering price, for sale to certain individuals through a directed share program, including our directors, certain employees and certain other individuals identified by management. Shares purchased through the directed share program will not be subject to a lock-up restriction, except in the case of shares purchased by any of our directors or officers and certain of our employees and existing equityholders. The number of shares of our Class A common stock available for sale to the general public will be reduced to the extent these individuals or entities purchase such reserved shares. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same basis as the other shares offered by this prospectus. | |
| Dividend policy |
We currently intend to retain any future earnings for investment in our business and do not expect to pay any dividends in the foreseeable future. The declaration and payment of all future dividends, if any, will be at the discretion of our board of directors (our “Board”) and will depend upon our financial condition, earnings, contractual conditions or applicable laws and other factors that our Board may deem relevant. See “Dividend Policy.” | |
18
| Exchange rights of holders of the LLC Units |
Prior to this offering, we will enter into the Exchange Agreement with MLSH 1 so that it may exchange LLC Units, together with an equal number of shares of Class B common stock, for shares of Class A common stock on a one-for-one basis or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). Any shares of Class B common stock so delivered will be cancelled. See “Organizational Structure—Exchange Agreement.” | |
| Tax Receivable Agreement |
We will enter into the Tax Receivable Agreement with MLSH 1 and MLSH 2 that will provide for the payment by us to MLSH 1 and MLSH 2, collectively, of 85% of the amount of tax benefits, if any, that Maravai LifeSciences Holdings, Inc. actually realizes (or in some circumstances is deemed to realize) as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement See “Organizational Structure—Tax Receivable Agreement.” | |
| Registration Rights Agreement |
We intend to enter into a registration rights agreement (the “Registration Rights Agreement”) with MLSH 1 and MLSH 2 in connection with this offering. The Registration Rights Agreement will provide MLSH 1 and MLSH 2 certain registration rights whereby, following our initial public offering and the expiration of any related lock-up period, MLSH 1 and MLSH 2 can require us to register under the Securities Act shares of Class A common stock, (including shares issuable to MLSH 1 upon exchange of its LLC Units). The Registration Rights Agreement will also provide for piggyback registration rights for MLSH 1 and MLSH 2. See “Certain Relationships and Related Party Transactions—Registration Rights Agreement.” | |
| Risk factors |
Investing in our Class A common stock involves a high degree of risk. See “Risk Factors” elsewhere in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our Class A common stock. | |
| Symbol for trading on The Nasdaq Global Select Market |
“MRVI.” | |
19
Unless otherwise indicated, all information in this prospectus:
| • | assumes the effectiveness of the Organizational Transactions; |
| • | assumes an initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover of this prospectus; |
| • | assumes that the underwriters’ option to purchase additional shares of Class A common stock is not exercised; |
| • | includes a 253,916.941-for-1 split of LLC Units, which was effected on November 11, 2020; |
| • | excludes the shares of Class A common stock that may be issuable upon exercise of redemption and exchange rights held by MLSH 1; |
| • | excludes 10,313,540 shares of Class A common stock, plus future increases, reserved for issuance under our 2020 Employee Stock Purchase Plan (the “ESPP”); and |
| • | excludes 25,783,851 shares of Class A common stock reserved for future issuance under the 2020 Plan, including (i) options to purchase 1,584,400 shares of Class A common stock that we will issue to certain employees upon completion of this offering, with an exercise price set at the initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover of this prospectus, and that vest in accordance with the schedule described in “Executive Compensation—Actions Taken in 2020 or in Connection with This Offering—Stock Option Grants” and (ii) 75,294 restricted stock units (“RSUs”) that may be settled for an equal number of shares of Class A common stock that we will issue to our six independent directors upon completion of this offering and that vest annually over three years. |
20
SUMMARY HISTORICAL FINANCIAL AND OTHER DATA
The following tables present, as of the dates and for the periods indicated, (1) the summary historical consolidated financial and other data for Topco LLC and its consolidated subsidiaries and (2) the summary unaudited pro forma financial data for Maravai LifeSciences Holdings, Inc. and its consolidated subsidiaries, including Topco LLC. Topco LLC is the predecessor of Maravai LifeSciences Holdings, Inc. for financial reporting purposes. The summary condensed consolidated statement of operations data for the nine months ended September 30, 2019 and 2020 and the summary condensed consolidated balance sheet data as of September 30, 2020 have been derived from the unaudited condensed consolidated financial statements and notes of Topco LLC and its subsidiaries included elsewhere in this prospectus. The summary consolidated statement of operations data for the years ended December 31, 2018 and 2019 and the summary consolidated balance sheet data as of December 31, 2018 and 2019 have been derived from the audited consolidated financial statements and notes of Topco LLC and its subsidiaries included elsewhere in this prospectus.
The results of operations for the periods presented below are not necessarily indicative of the results to be expected for any future period and the results for any interim period are not necessarily indicative of the results that may be expected for a full fiscal year. The information set forth below should be read together with “Use of Proceeds,” “Capitalization,” “Selected Consolidated Financial Data,” “Unaudited Pro Forma Condensed Consolidated Financial Information” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
The summary unaudited pro forma consolidated financial data of Maravai LifeSciences Holdings, Inc. presented below have been derived from our unaudited pro forma condensed consolidated financial statements and notes included elsewhere in this prospectus. The summary unaudited pro forma financial data as of and for the year ended December 31, 2019 and as of and for the nine months ended September 30, 2020, gives effect to the Organizational Transactions as described in “Organizational Structure,” including the consummation of this offering, the use of the net proceeds therefrom and related transactions, as described in “Use of Proceeds” and “Unaudited Pro Forma Condensed Consolidated Financial Data,” as if all such transactions had occurred on January 1, 2019, with respect to the condensed consolidated statement of operations data and September 30, 2020, with respect to the condensed consolidated balance sheet data. The unaudited pro forma financial data include various estimates that are subject to material change and may not be indicative of what our operations or financial position would have been had this offering and related transactions taken place on the dates indicated, or that may be expected to occur in the future. See “Unaudited Pro Forma Condensed Consolidated Financial Information” for a complete description of the adjustments and assumptions underlying the summary unaudited pro forma consolidated financial data.
21
The summary historical consolidated financial and other data of Maravai LifeSciences Holdings, Inc. have not been presented as Maravai LifeSciences Holdings, Inc. is a newly incorporated entity, has had no business transactions or activities to date and had no material assets or liabilities during the periods presented in this section.
| Historical Topco LLC | Pro Forma Maravai LifeSciences Holdings, Inc. |
|||||||||||||||||||||||
| (in thousands, except per share and per unit data) |
Year Ended December 31, | Nine Months Ended September 30, |
Year Ended December 31, 2019 |
Nine Months Ended September 30, 2020 |
||||||||||||||||||||
| 2018 | 2019 | 2019 | 2020 | |||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||||||
| Revenue |
$ | 123,833 | $ | 143,140 | $ | 107,180 | $ | 185,745 | $ | 143,140 | $ | 185,745 | ||||||||||||
| Operating Expenses: |
||||||||||||||||||||||||
| Cost of revenue |
60,765 | 66,849 | 49,019 | 56,254 | 66,849 | 56,254 | ||||||||||||||||||
| Research and development |
4,499 | 3,627 | 2,648 | 7,212 | 3,627 | 7,212 | ||||||||||||||||||
| Selling, general and administrative |
41,194 | 48,354 | 32,570 | 52,624 | 57,586 | 58,329 | ||||||||||||||||||
| Change in estimated fair value of contingent consideration |
939 | 322 | 241 | — | 322 | — | ||||||||||||||||||
| Gain on sale and leaseback transaction |
— | — | — | (19,002 | ) | — | (19,002 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
107,397 | 119,152 | 84,478 | 97,088 | 128,384 | 102,793 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income from operations |
16,436 | 23,988 | 22,702 | 88,657 | 14,756 | 82,952 | ||||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||
| Interest expense |
(27,399 | ) | (29,959 | ) | (22,240 | ) | (21,934 | ) | (29,165 | ) | (22,980 | ) | ||||||||||||
| Loss on extinguishment of debt |
(5,622 | ) | — | — | — | — | — | |||||||||||||||||
| Other income |
87 | 118 | 95 | 132 | 118 | 132 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (Loss) income before income taxes |
(16,498 | ) | (5,853 | ) | 557 | 66,855 | (14,291 | ) | 60,104 | |||||||||||||||
| Income tax expense (benefit) |
417 | (652 | ) | 308 | 2,511 | (1,559 | ) | 6,015 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (loss) income |
$ | (16,915 | ) | $ | (5,201 | ) | $ | 249 | $ | 64,344 | (12,732 | ) | 54,089 | |||||||||||
| Net (loss) income attributable to noncontrolling interests |
(12,443 | ) | (731 | ) | (672 | ) | 582 | (9,335 | ) | 39,415 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (loss) income attributable to Topco LLC member |
$ | (4,472 | ) | $ | (4,470 | ) | $ | 921 | $ | 63,762 | (3,397 | ) | $ | 14,674 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (loss) income per common unit attributable to Topco LLC member—basic and diluted |
$ | (0.07 | ) | $ | (0.03 | ) | $ | (0.01 | ) | $ | 0.21 | |||||||||||||
| Weighted-average common units outstanding |
253,916,941 | 253,916,941 | 253,916,941 | 253,916,941 | ||||||||||||||||||||
| Per Share Data(1): |
||||||||||||||||||||||||
| Pro forma weighted average shares of Class A common stock outstanding: |
||||||||||||||||||||||||
| Basic |
|
84,465,072 | 84,465,072 | |||||||||||||||||||||
| Diluted |
|
84,465,072 | 84,499,582 | |||||||||||||||||||||
| Pro forma net loss available to Class A common stock per share: |
||||||||||||||||||||||||
| Basic |
|
$ | (0.04 | ) | $ | 0.18 | ||||||||||||||||||
| Diluted |
|
$ | (0.04 | ) | $ | 0.18 | ||||||||||||||||||
| Selected Other Data: |
||||||||||||||||||||||||
| Adjusted EBITDA(2) |
$ | 53,000 | $ | 62,014 | $ | 48,150 | $ | 104,840 | ||||||||||||||||
| Adjusted Free Cash Flow(3) |
$ | 49,263 | $ | 42,101 | $ | 34,713 | $ | 83,118 | ||||||||||||||||
22
| Historical Topco LLC | Pro Forma Maravai LifeSciences Holdings, Inc. |
|||||||||||||||
| As of December 31, | As of September 30, | As of September 30, 2020 |
||||||||||||||
| 2018 | 2019 | 2020 | ||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Consolidated Balance Sheet Data (at period end): |
||||||||||||||||
| Cash |
$ | 21,866 | $ | 24,700 | $ | 124,882 | $ | 171,657 | ||||||||
| Working capital (deficit)(4) |
17,883 | 30,990 | (46,330 | ) | 157,341 | |||||||||||
| Total assets |
539,676 | 577,796 | 738,112 | 1,093,031 | ||||||||||||
| Long-term debt, less current portion |
335,550 | 334,783 | 349,204 | 530,100 | ||||||||||||
| Total liabilities |
391,660 | 433,169 | 702,118 | 1,003,340 | ||||||||||||
| Total member’s/shareholders’ equity |
148,016 | 144,627 | 35,994 | 89,691 | ||||||||||||
| (1) | See the unaudited pro forma consolidated statement of operations in “Unaudited Pro Forma Consolidated Financial Information” for the description of the assumptions underlying the pro forma net loss per share calculations. |
| (2) | Adjusted EBITDA is a supplemental measure of operating performance that is not prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) and that does not represent, and should not be considered as, an alternative to net loss, as determined in accordance with GAAP. We define Adjusted EBITDA as net loss before interest, taxes, depreciation and amortization, certain non-cash items and other adjustments that we do not consider in our evaluation of ongoing operating performance from period to period. |
We use Adjusted EBITDA to understand and evaluate our core operating performance and trends and to develop short-term and long-term operating plans. We believe that Adjusted EBITDA facilitates comparison of our operating performance on a consistent basis between periods and, when viewed in combination with our results prepared in accordance with GAAP, helps provide a broader picture of factors and trends affecting our results of operations.
Adjusted EBITDA has limitations as an analytical tool, and you should not consider it in isolation, or as a substitute for analysis of our results as reported under GAAP. Because of these limitations, Adjusted EBITDA should not be considered as a replacement for net loss, as determined by GAAP, or as a measure of our profitability. We compensate for these limitations by relying primarily on our GAAP results and using non-GAAP measures only for supplemental purposes.
A reconciliation of Adjusted EBITDA to net loss, the most directly comparable GAAP measure, is as follows:
| Historical Topco LLC | ||||||||||||||||
| (in thousands) | Year Ended December 31, |
Nine Months Ended September 30, |
||||||||||||||
| 2018 | 2019 | 2019 | 2020 | |||||||||||||
| Net income (loss) |
$ | (16,915 | ) | $ | (5,201 | ) | $ | 249 | $ | 64,344 | ||||||
| Add: |
||||||||||||||||
| Amortization |
20,122 | 20,274 | 15,118 | 15,156 | ||||||||||||
| Depreciation |
2,225 | 3,810 | 2,331 | 4,756 | ||||||||||||
| Interest expense |
27,399 | 29,959 | 22,240 | 21,934 | ||||||||||||
| Income tax expense (benefit) |
417 | (652 | ) | 308 | 2,511 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| EBITDA |
33,248 | 48,190 | 40,246 | 108,701 | ||||||||||||
23
| Historical Topco LLC | ||||||||||||||||
| (in thousands) | Year Ended December 31, |
Nine Months Ended September 30, |
||||||||||||||
| 2018 | 2019 | 2019 | 2020 | |||||||||||||
| Acquisition contingent consideration(a) |
939 | 322 | 241 | — | ||||||||||||
| Loss on extinguishment of debt(b) |
5,622 | — | — | — | ||||||||||||
| Acquisition integration costs(c) |
7,529 | 6,170 | 4,061 | 3,588 | ||||||||||||
| Amortization of purchase accounting inventory step-up(d) |
2,967 | 1,856 | 1,856 | — | ||||||||||||
| Acquired in-process research and development costs(e) |
— | — | — | 2,881 | ||||||||||||
| Unit-based compensation(f) |
2,121 | 1,679 | 1,163 | 2,933 | ||||||||||||
| GTCR management fees(g) |
574 | 523 | 421 | 555 | ||||||||||||
| Gain on sale and leaseback transaction(h) |
— | — | — | (19,002 | ) | |||||||||||
| Merger and acquisition related expenses(i) |
— | 3,274 | 162 | 218 | ||||||||||||
| Financing costs(j) |
— | — | — | 4,966 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Adjusted EBITDA |
$ | 53,000 | $ | 62,014 | $ | 48,150 | $ | 104,840 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (a) | Refers to the change in fair value and settlement of earn-out payments related to a 2017 acquisition. |
| (b) | Refers to non-operating cash expense incurred on extinguishment of debt. |
| (c) | Refers to incremental costs incurred to execute and integrate completed acquisitions. |
| (d) | Refers to a non-cash charge related to the amortization expense of the step-up of inventory from purchase price accounting. |
| (e) | Refers to an in-process research and development charge associated with the acquisition of MockV Solutions, Inc. |
| (f) | Refers to non-cash expense associated with unit-based compensation. |
| (g) | Refers to cash fees paid to GTCR pursuant to the advisory services agreement that will terminate in connection with this offering. |
| (h) | Refers to the gain on the sale of our Burlingame, California facility, which was leased back to the Company in 2020. |
| (i) | Refers to diligence, legal, accounting, tax and consulting fees incurred associated with an acquisition that was not consummated. |
| (j) | Refers to transaction costs related to this offering and the refinancing of our Credit Facilities that are not capitalizable or cannot be offset against proceeds from such transactions. |
| (3) | Adjusted Free Cash Flow is a supplemental measure of operating performance that is not prepared in accordance with GAAP and that does not represent, and should not be considered as, an alternative to net loss, as determined in accordance with GAAP. We define Adjusted Free Cash Flow as Adjusted EBITDA less capital expenditures. |
We believe that Free Cash Flow facilitates comparison of our operating performance on a consistent basis between periods and, when viewed in combination with our results prepared in accordance with GAAP, helps provide a broader picture of factors and trends affecting our results of operations.
Adjusted Free Cash Flow has limitations as an analytical tool, and you should not consider it in isolation, or as a substitute for an analysis of our results as reported under GAAP. Because of these limitations, Adjusted Free Cash Flow should not be considered as a replacement for net loss, as determined by GAAP, or as a measure of our profitability. We compensate for these limitations by relying primarily on our GAAP results and using non-GAAP measures only for supplemental purposes.
24
A reconciliation of Adjusted Free Cash Flow is as follows:
| Historical Topco LLC | ||||||||||||||||
| Year Ended December 31, |
Nine Months Ended September 30, |
|||||||||||||||
| 2018 | 2019 | 2019 | 2020 | |||||||||||||
| Adjusted EBITDA |
$ | 53,000 | $ | 62,014 | $ | 48,150 | $ | 104,840 | ||||||||
| Capital expenditures(a) |
(3,737 | ) | (19,913 | ) | (13,437 | ) | (21,722 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Adjusted Free Cash Flow |
$ | 49,263 | $ | 42,101 | $ | 34,713 | $ | 83,118 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (a) | We define capital expenditures as purchases of property and equipment, which are included in cash flows from investing activities, and accounts payable and accrued expenses. |
| (4) | We define working capital (deficit) as current assets less current liabilities. |
25
Investing in our Class A common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information contained in this prospectus, including our consolidated financial statements and the related notes thereto, before making a decision to invest in our Class A common stock. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. If any of the following risks occur, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that event, the price of our Class A common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business and Strategy
Until the 2020 fiscal year, we had incurred losses for each fiscal year since inception, we may incur losses in the future and we may not be able to generate sufficient revenue to maintain profitability.
Until the 2020 fiscal year, we had incurred losses for each fiscal year since our inception. For the years ended December 31, 2018 and 2019, we incurred net losses of $16.9 million and $5.2 million, respectively. As of December 31, 2019, we had an accumulated deficit of $42.4 million. Although we generated net income of $64.3 million for the nine months ended September 30, 2020 and had retained earnings of $21.4 million as of September 30, 2020, we expect that our operating expenses will continue to increase as we grow our business and as a result of our becoming a public company, and we may be unable to maintain profitability for the fiscal year ending December 31, 2020 or any future period. Since our inception, we have financed our operations primarily through the incurrence of indebtedness, revenue from our products and services and the sale of our equity securities. We will need to generate significant additional revenue to maintain profitability and we cannot be sure that we will remain profitable for any substantial period of time. We may never be able to generate sufficient revenue to maintain profitability and our recent and historical growth should not be considered indicative of our future performance.
Our operating results may fluctuate significantly in the future, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide.
Our quarterly and annual operating results may fluctuate significantly, which makes it difficult for us to predict our future operating results. These fluctuations may be driven by a variety of factors, many of which are outside of our control, including, but not limited to:
| • | demand from our largest customers, which account for a significant percentage of our sales and orders, may not meet our expectations regarding volume and price in any given time period; |
| • | the level of demand for our products and services, which may vary significantly, and our ability to increase penetration in our existing markets and expand into new markets; |
| • | customers accelerating, canceling, reducing or delaying orders as a result of developments related to their pre-clinical studies and clinical trials; |
| • | impacts on us, our suppliers and our customers as a result of the COVID-19 pandemic; |
| • | the relative reliability and robustness of our products and services; |
| • | changes in governmental regulations or the regulatory posture toward our business; |
| • | the volume and mix of the products and services we sell or changes in the production or sales costs related to our products and services; |
| • | the success of our newer products, such as our CleanCap® mRNA products, and the introduction of other new products or product enhancements by us or others in our industry; |
26
| • | the timing and amount of expenditures that we may incur to acquire, develop or commercialize additional products, services and technologies or for other purposes, such as the expansion of our facilities; |
| • | changes in governmental and academic funding of life sciences research and developments or changes that impact budgets, budget cycles or seasonal spending patterns of our customers; |
| • | future accounting pronouncements or changes in our accounting policies; |
| • | difficulties encountered by our commercial carriers in delivering our products, whether as a result of external factors such as weather or internal issues such as labor disputes; |
| • | general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors; and |
| • | the other factors described in this “Risk Factors” section. |
The impact of any one of the factors discussed above, or the cumulative effects of a combination of such factors, could result in significant fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparisons of our operating results on a period-to-period basis may not be meaningful. Investors should not rely on our past results as an indication of our future performance.
As a result of variability and unpredictability, we may also fail to meet the expectations of industry or financial analysts or investors for any period. If our revenue or operating results fall short of the expectations of analysts or investors or any guidance we may provide, or if the guidance we provide falls short of the expectations of analysts or investors, the price of our Class A common stock could decline substantially. Such a stock price decline could occur even when we have met or exceeded any previously publicly stated guidance we may have provided.
We depend on a limited number of customers for a high percentage of our revenue. If we cannot maintain our current relationships with customers, fail to sustain recurring sources of revenue with our existing customers, or if we fail to enter into new relationships, our future operating results will be adversely affected.
For the years ended December 31, 2018 and 2019, revenue from our ten largest customers accounted for 33% and 34% of our total revenue, respectively. For the years ended December 31, 2018 and 2019, revenue from our five largest customers accounted for 25% and 26% of our total revenue, respectively, and revenue from our sixth to tenth largest customers accounted for 8% and 9% of our total revenue during the same periods, respectively. For the nine months ended September 30, 2020, revenue from our ten largest, five largest and sixth to tenth largest customers accounted for 53%, 43% and 9% of our total revenue, respectively. As of December 31, 2018, one single customer represented 13% of total consolidated accounts receivable. As of December 31, 2019, two customers accounted for 11% and 10% of total consolidated accounts receivable. Our largest customer, Thermo Fisher Scientific Inc., accounted for 9% and 10% of our total revenue for the years ended December 31, 2018 and 2019, respectively. As of September 30, 2020, two customers accounted for 52% and 14% of total consolidated accounts receivable. Our largest customer, Pfizer Inc., accounted for 14% of our total revenue for the nine months ended September 30, 2020. No other customer has accounted for 10% or more of our total revenue for these periods. The revenue attributable to our top customers has fluctuated in the past and may fluctuate in the future, which could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects. In addition, the termination of these relationships, including following any failure to renew a long-term contract, could result in a temporary or permanent loss of revenue.
Our future success depends on our ability to maintain these relationships, to increase our penetration among these existing customers and to establish new relationships. We engage in conversations with other companies and institutions regarding potential commercial opportunities on an ongoing basis, which can be time consuming. There is no assurance that any of these conversations will result in a commercial agreement, or if an agreement is reached, that the resulting relationship will be successful. Speculation in the industry about our existing or potential commercial relationships can be a catalyst for adverse speculation about us, our products, our services and our technology, which can adversely affect our reputation and our business. In addition, if our customers
27
order our products or services, but fail to pay on time or at all, our liquidity, financial condition, results of operations, cash flows and prospects could be materially and adversely affected.
We cannot assure investors that we will be able to further penetrate our existing markets or that our products or services will gain adequate market acceptance. Any failure to increase penetration in our existing markets would adversely affect our ability to improve our operating results.
Certain of our products are used by customers in the production of vaccines and therapies, some of which represent relatively new and still-developing modes of treatment. Unforeseen adverse events, negative clinical outcomes, or increased regulatory scrutiny of these and their financial cost may damage public perception of the safety, utility, or efficacy of these vaccines and therapies or other modes of treatment and may harm our customers’ ability to conduct their business. Such events may negatively impact our revenue and have an adverse effect on our performance.
Gene therapy and nucleic acid vaccines remain relatively new and are under active development, with only a few gene therapies and no nucleic acid vaccines approved to date by regulatory authorities. Public perception may be influenced by claims that gene therapy or nucleic acid vaccines are unsafe or ineffective, and gene therapy may not gain the acceptance of the public or the medical community. In addition, ethical, social, legal and financial concerns about gene therapy and nucleic acid vaccines could result in additional regulations or limitations or even prohibitions on certain gene therapies or vaccine-related products. More restrictive regulations or negative public perception could reduce certain of our customers’ use of our products and services, which could negatively affect our revenue and performance. In addition, certain of the COVID-19 vaccine development programs that may incorporate our CleanCap® products are still in early stages of development, including those with Chula Vaccine Research Center in partnership with the University of Pennsylvania, eTheRNA Immunotherapies and Greenlight Biosciences. There can be no assurance that these vaccine programs will proceed to clinical trials or result in a commercial product, or that any resulting vaccine will incorporate our CleanCap® products.
A pandemic, epidemic, or outbreak of an infectious disease, such as COVID-19, has affected, and may continue to materially and adversely affect our business, financial condition, results of operations, cash flows and prospects.
In late 2019, COVID-19 surfaced in Wuhan, China. Since then, COVID-19 has spread to multiple other regions and countries, including the San Francisco Bay Area, where our protein detection business is located, the San Diego, California and Washington, D.C. areas, where our nucleic acid production business is located and the Wilmington, North Carolina area, where our biologics safety testing products business is located. The COVID-19 pandemic is evolving and to date has led to the implementation of various responses, including government imposed shelter-in-place orders, quarantines, travel restrictions and other public health safety measures, as well as reported adverse impacts on healthcare resources, facilities and providers in California, across the United States and in other countries. In response to the spread of COVID-19, and in accordance with direction from state and local government authorities, we have restricted access to our facilities mostly to personnel and third parties who must perform critical activities that must be completed on-site, limited the number of such personnel that can be present at our facilities at any one time, and requested that many of our personnel work remotely. In the event that government authorities were to further modify current restrictions, our employees conducting research and development or manufacturing activities may not be able to access our laboratory or manufacturing facilities and our core activities may be significantly limited or curtailed, possibly for an extended period of time.
As a result of the COVID-19 pandemic, or similar pandemics and outbreaks that may occur in the future, we have experienced and may in the future experience severe disruptions, including:
| • | interruption of or delays in receiving products and supplies from the third parties we rely on to, among other things, manufacture components to our products, due to staffing shortages, production slowdowns or stoppages and disruptions in delivery systems, which may impair our ability to manufacture and sell our products and services; |
28
| • | limitations on our business operations by the local, state or federal government that could impact our ability to manufacture, sell or deliver our products and services; |
| • | on-site visit limitations and prohibitions imposed by customers that could impact our ability to engage in pre-sales activities, and to provide post-sale activities, such as training, service and support; |
| • | delays in customers’ purchasing decisions and negotiations with customers and potential customers; |
| • | business disruptions caused by workplace, laboratory and office closures and an increased reliance on employees working from home, travel limitations, cyber security and data accessibility limits, or communication or mass transit disruptions; and |
| • | limitations on employee resources that would otherwise be focused on the conduct of our activities, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people. |
Any of these factors could severely impact our research and development activities, manufacturing business operations and sales or delay necessary interactions with local regulators, third-party vendors and other important contractors and customers. These and other factors arising from the COVID-19 pandemic could worsen in countries that are already experiencing significant levels of COVID-19 infections, could continue to spread to additional countries or could return to countries where the pandemic has been partially contained and could further adversely impact our ability to conduct our business generally and have a material adverse impact on our business, financial condition, results of operations, cash flows and prospects. For example, our protein detection segment experienced a decrease in sales for the second quarter of 2020 relative to the same period in 2019 due to stay-at-home orders in the San Francisco Bay Area and the closure of many academic laboratories that are the main customers of this segment and the reduced operations of other customers. Prolonged closures or shutdowns as a result of the COVID-19 pandemic would continue to affect sales of our protein detection segment adversely.
The extent to which the pandemic may negatively impact our consolidated operations and results of operations or those of our third-party manufacturers, suppliers, partners or customers will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the duration of the pandemic, the extent of travel restrictions, additional or modified government actions, new information that will emerge concerning the severity and impact of COVID-19 and actions to contain the pandemic or treat its impact, such as social distancing, quarantines, lock-downs or business closures.
We cannot presently predict the scope and severity of any potential business shutdowns or disruptions as a result of the COVID-19 pandemic. If we or any of the third parties with whom we engage were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted, which could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Changes in economic conditions could negatively impact our revenue and earnings.
Our reagents are sold primarily to biopharmaceutical and academic organizations developing novel vaccines and therapies and performing basic research. Research and development spending by our customers and the availability of government research funding can fluctuate due to changes in available resources, mergers of pharmaceutical and biotechnology companies, spending priorities, general economic conditions and institutional and governmental budgetary policies. Our biologics safety testing customers are biopharmaceutical companies, contract research organizations (“CROs”), contract development and manufacturing organizations (“CDMOs”) and life science companies, which largely serve the biopharmaceutical industry. Our nucleic acid production customers are largely vaccine and therapeutic drug makers or diagnostics manufacturers, which rely in part on government healthcare-related policies and funding. Changes in government funding for certain research or reductions in overall healthcare spending could negatively impact us or our customers and, correspondingly, our
29
sales to them. Currently, the U.S. and global economies are experiencing a period of economic downturn as a result of the COVID-19 pandemic. Other global economies have been slow to recover from past downturns. Any continued or further economic downturns or reductions or delays in governmental funding could cause customers to delay or forego purchases of our products and services. In addition, the majority of our customers’ contracts can be terminated, delayed or reduced in scope upon short notice or no notice. Changes in the level of orders received and filled can cause fluctuations in our quarterly revenue and earnings.
We are dependent on our customers’ spending on and demand for outsourced nucleic acid production, biologics safety testing and protein detection research products and services. A reduction in spending or demand could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
The success of our business depends primarily on the number and size of contracts with our customers, primarily pharmaceutical and biotechnology companies, for our products and services. Over the past several years, we have benefited from an increased demand for our products and services as a result of the continued growth of the global biologics market, increasing research and development budgets of our customers and greater degree of outsourcing by our customers. A slowing or reversal of any of these trends could have a significant adverse effect on the demand for our products and services.
In addition to these industry trends, our customers’ willingness and ability to utilize our products and services are also subject to, among other things, their own financial performance, changes in their available resources, their decisions to acquire in-house manufacturing capacity, their spending priorities, their budgetary policies and practices and their need to develop new biological products, which, in turn, are dependent upon a number of factors, including their competitors’ discoveries, developments and commercial manufacturing initiatives and the anticipated market, clinical and reimbursement scenarios for specific products and therapeutic areas. In addition, consolidation in the industries in which our customers operate may have an impact on our customers’ spending as they integrate acquired operations, including research and development departments and associated budgets. If our customers reduce their spending on our products and services as a result of any of these or other factors, our business, financial condition, results of operations, cash flows and prospects would be materially and adversely affected.
We compete with life science, pharmaceutical and biotechnology companies who are substantially larger than we are and potentially capable of developing new approaches that could make our products, services and technology obsolete.
The market for pharmaceutical, reagent, therapeutic and diagnostic products and services is intensely competitive, rapidly evolving, significantly affected by new product introductions and other market activities by industry participants and subject to rapid technological change. We also expect increased competition as additional companies enter our market and as more advanced technologies become available. We compete with other providers of outsourced biologics products and services. We also compete with the in-house discovery, development and commercial manufacturing functions of pharmaceutical and biotechnology companies. Many of our competitors are large, well-capitalized companies with significantly greater resources and market share than we have. As a consequence, these competitors are able to spend more aggressively on product and service development, marketing, sales and other initiatives than we can. Many of these competitors also have:
| • | broader name recognition; |
| • | longer operating histories and the benefits derived from greater economies of scale; |
| • | larger and more established distribution networks; |
| • | additional product and service lines and the ability to bundle products and services to offer higher discounts or other incentives to gain a competitive advantage; |
| • | more experience in conducting research and development, manufacturing and marketing; |
30
| • | more experience in entering into collaborations or other strategic partnership arrangements; and |
| • | more financial, manufacturing and human resources to support product development, sales and marketing and patent and other intellectual property litigation. |
These factors, among others, may enable our competitors to market their products and services at lower prices or on terms more advantageous to customers than we can offer. Competition may result in price reductions, reduced gross margins and loss of market share, any of which could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects. Additionally, our current and future competitors, including certain of our customers, may at any time develop additional products and services that compete with our products and services and new approaches by these competitors may make our products, services, technologies and methodologies obsolete or noncompetitive. We may not be able to compete effectively against these organizations.
In addition, to develop and market our new products, services, technologies and methodologies successfully, we must accurately assess and meet customers’ needs, make significant capital expenditures, optimize our development and manufacturing processes to predict and control costs, hire, train and retain the necessary personnel, increase customer awareness and acceptance of our services, provide high-quality services in a timely manner, price our products and services competitively and effectively integrate customer feedback into our business planning. If we fail to create demand for our new products, services or technologies, our future business could be harmed.
If our products and services do not perform as expected or the reliability of the technology on which our products and services are based is questioned, we could experience lost revenue, delayed or reduced market acceptance of our products and services, increased costs and damage to our reputation.
Our success depends on the market’s confidence that we can provide reliable, high-quality life science reagents. We believe that customers in our target markets are likely to be particularly sensitive to product defects and errors. Our reputation and the public image of our products, services and technologies may be impaired if our products or services fail to perform as expected.
Although our products are tested prior to shipment, defects or errors could nonetheless occur. Our operating results depend on our ability to execute and, when necessary, improve our quality management strategy and systems and our ability to effectively train and maintain our employee base with respect to quality management. A failure of our quality control systems could result in problems with facility operations or preparation or provision of products. In each case, such problems could arise for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with raw materials or environmental factors and damage to, or loss of, manufacturing operations. Such problems could affect production of a particular batch or series of batches of products, requiring the destruction of such products or a halt of facility production altogether. Furthermore, some of the products that we manufacture are subsequently incorporated into products that are sold by other life sciences companies and we have no control over the manufacture and production of those products.
In addition, in the event we, or our suppliers, fail to meet required quality standards and if our products experience, or are perceived to experience, a material defect or error, our products could be recalled or we may be unable to timely deliver products to our customers, which in turn could damage our reputation for quality and service. In the past, certain of our custom mRNA and CleanCap® reagent products have been sold with insufficient capping efficiency or with incorrect transcription instructions. Additionally, several lots of our HCP ELISA biologics safety testing kits have experienced a possible instability drift and decrease in accuracy. Although we have taken steps to improve our quality review, product documentation and reference testing procedures, we cannot guarantee that we will not experience quality assurance issues with our products in the future. Any such failure could, among other things, lead to increased costs, delayed or lost revenue, delayed
31
market acceptance, damaged reputation, diversion of development resources, legal claims, reimbursement to customers for lost drug product, starting materials and active pharmaceutical ingredients, other customer claims, damage to and possibly termination of existing customer relationships, increased insurance costs, time and expense spent investigating the cause and, depending on the cause, similar losses with respect to other batches or products, any of which could harm our business, financial condition, results of operations, cash flows and prospects. Such defects or errors could also narrow the scope of the use of our products, which could hinder our success in the market.
Even after any underlying concerns or problems are resolved, any lingering concerns in our target markets regarding our technology or any manufacturing defects or performance errors in our products or services could continue to result in lost revenue, delayed market acceptance, damage to our reputation and claims against us.
In addition, we may be unable to maintain the quality, reliability, robustness and expected turnaround times of our products and services to continue to satisfy customer demand as we grow. To effectively manage our growth, we must continue to improve our operational, manufacturing and quality control systems and processes and other aspects of our business and continue to effectively expand, train and manage our personnel. The time and resources required to improve our existing systems and procedures, implement new systems and procedures and to adequately staff such existing and new systems and procedures is uncertain, and failure to complete this in a timely and efficient manner could adversely affect our operations and negatively impact our business and financial results. We may need to purchase additional equipment, some of which can take several months or more to procure, set up and validate, establish new production processes and increase our personnel levels to meet increased demand. There can be no assurance that any of these increases in scale, personnel expansion or equipment or process enhancements will be successfully implemented, or that we will have adequate space, including in our laboratory and production facilities, to accommodate such required expansion. Failure to manage this growth or transition could result in delays in turnaround times, higher product costs, declining product quality, deteriorating customer service and slower responses to competitive challenges. A failure in any one of these areas could make it difficult for us to meet market expectations for our products and services and could damage our reputation and our business, financial condition, results of operations, cash flows and prospects could be adversely affected.
Our products are highly complex and are subject to quality control requirements.
Whether a product is produced by us or purchased from outside suppliers, it is subject to quality control procedures, including the verification of stability and performance and, for certain products, additional validation required by certain GMP that we voluntarily follow, European Conformity (“CE”) marking and ISO 9001:2015 compliance, prior to final packaging. Certain of our products are manufactured following the voluntary GMP quality standards of the International Council for Harmonisation’s GMP Guide, comparable GMP principles for the European Union and customer-specific requirements. We believe these products are exempt from compliance with the Food, Drug, and Cosmetic Act (“FDCA”) and the current GMP (“cGMP”) regulations of the Food and Drug Administration (“FDA”), as our products are further processed and incorporated into final drug products by our customers and we do not make claims related to their safety or effectiveness. In the event we, or our suppliers, produce products that fail to comply with required quality standards, we may incur delays in fulfilling orders, write-downs, damages resulting from product liability claims and harm to our reputation.
We rely on a limited number of suppliers or, in some cases, sole suppliers, for some of our raw materials and may not be able to find replacements or immediately transition to alternative suppliers.
Certain of our raw materials are sourced from a limited number of suppliers and some materials, including a proprietary DNA reagent, certain packaging materials, specific cell lines for Cygnus Technologies’ operations and certain raw materials used in our nucleic acid production products, as well as those raw materials sold under the Glen Research brand, are sole sourced. Delays or difficulties in securing these raw materials or other laboratory materials could result in an interruption in our production operations if we cannot obtain an acceptable
32
substitute. Any such interruption could significantly affect our business, financial condition, results of operations, cash flows and prospects. While we may identify other suppliers, raw materials furnished by such replacement suppliers may require us to alter our production operations or perform extensive validations, which may be time consuming and expensive. There can be no assurance that we will be able to secure alternative materials and revalidate them without experiencing interruptions in our workflow. If we should encounter delays or difficulties in obtaining raw materials, our business, financial condition, results of operations, cash flows and prospects could be adversely affected.
We depend on a stable and adequate supply of quality raw materials from our suppliers, and price increases or interruptions of such supply could have an adverse impact on our business, financial condition, results of operations, cash flows and prospects.
Our operations depend upon our ability to obtain raw materials at reasonable prices. If we are unable to obtain the materials we need at a reasonable price, we may not be able to produce certain of our products at marketable prices or at all, which could have a material adverse effect on our results of operations.
Although we believe that we have stable relationships with our existing suppliers, we cannot assure you that we will be able to secure a stable supply of raw materials going forward. Our suppliers may not be able to keep up with our pace of growth or may reduce or cease their supply of raw materials to us at any time. In addition, we cannot assure you that our suppliers have obtained and will be able to obtain or maintain all licenses, permits and approvals necessary for their operations or comply with all applicable laws and regulations, and failure to do so by them may lead to interruption in their business operations, which in turn may result in shortages of raw materials supplied to us. Some of our suppliers are based overseas and therefore may need to maintain export or import licenses. If the supply of raw materials is interrupted, our business, financial condition, results of operations, cash flows and prospects may be adversely affected.
Our business could be adversely affected by disruptions at our sites.
We rely upon our internal manufacturing, packaging and distribution operations to produce many of the products we sell and our warehouse facilities to store products pending sale. Any significant disruption of those operations for any reason, such as labor unrest, power interruptions, fire, hurricanes, the COVID-19 pandemic, earthquakes or other events beyond our control, could adversely affect our sales and customer relationships and therefore adversely affect our business. We have significant operations in California, near major earthquake faults, which make us susceptible to earthquake risk.
If we are unable to manufacture in specific quantities, our operating results will be harmed.
Our revenue and other operating results depend in large part on our ability to manufacture and ship our products in sufficient quantities. Any interruptions we experience in the manufacturing or shipping of our products could delay our ability to recognize revenue in a particular quarter. Manufacturing problems can and do arise, and as demand for our products increases, any such problems could have an increasingly significant impact on our operating results. While we have not generally experienced problems with, or delays in, our production capabilities that resulted in delays in our ability to ship finished products, there can be no assurance that we will not encounter such problems in the future. We may not be able to quickly ship products and recognize anticipated revenue for a given period if we experience significant delays in the manufacturing process. In addition, we must maintain sufficient production capacity in order to meet anticipated customer demand, and we may be unable to offset the associated fixed costs if orders slow, which would adversely affect our operating margins. If we are unable to manufacture our products consistently, in sufficient quantities and on a timely basis, our revenue, cash flow, gross margins and our other results of operations will be materially and adversely affected.
33
Natural disasters, geopolitical unrest, war, terrorism, public health issues or other catastrophic events could disrupt the supply, delivery or demand of products and services, which could negatively affect our operations and performance.
We are subject to the risk of disruption by earthquakes, hurricanes, floods and other natural disasters, fire, power shortages, geopolitical unrest, war, terrorist attacks and other hostile acts, public health issues, epidemics or pandemics, such as the COVID-19 pandemic, and other events beyond our control and the control of the third parties on which we depend. Any of these catastrophic events, whether in the United States or abroad, may have a significant negative impact on the global economy, our employees, facilities, partners, suppliers, distributors or customers, and could decrease demand for our products and services, create delays and inefficiencies in our supply chain and make it difficult or impossible for us to deliver products and services to our customers.
In addition, a catastrophic event that results in damage to specific equipment that would be difficult to replace, the destruction or disruption of our research and production facilities or our critical business or information technology systems would severely affect our ability to conduct normal business operations and, as a result, our operating results would be adversely affected.
Future strategic transactions or acquisitions may require us to seek additional financing, which we may not be able to secure on favorable terms, if at all.
We plan to continue a strategy of growth and development for our business. To this end, we actively evaluate various strategic transactions on an ongoing basis, including licensing or acquiring complementary products, technologies or businesses that would complement our existing portfolio of products and services. In order to complete such strategic transactions, we may need to seek additional financing to fund these investments and acquisitions. Should we need to do so, we may not be able to secure such financing, or obtain such financing on favorable terms because of the volatile nature of the biotechnology marketplace. In addition, future acquisitions may require the issuance or sale of additional equity, or equity-linked securities, which may result in additional dilution to our shareholders. Further, on October 19, 2020, we entered into a new credit agreement (the “New Credit Agreement”), which contains a number of restrictive covenants that impose significant restrictions on our ability to make acquisitions or certain other investments.
Because we rely heavily on third-party package-delivery services, a significant disruption in these services, damages or losses sustained during shipping or significant increases in prices could adversely affect our business, financial condition, results of operations, cash flows and prospects.
We ship a significant portion of our products to our customers through independent package delivery companies, such as FedEx, UPS and DHL. If one or more of these third-party package-delivery providers were to experience a major work stoppage, preventing our products from being delivered in a timely fashion or causing us to incur additional shipping costs we could not pass on to our customers, our costs could increase and our relationships with certain of our customers could be adversely affected. In addition, if one or more of these third-party package-delivery providers were to increase prices, and we were not able to find comparable alternatives or make adjustments in our delivery network, our profitability could be adversely affected. Furthermore, if one or more of these third-party package-delivery providers were to experience performance problems or other difficulties, it could negatively impact our operating results and our customers’ experience. In the past, some of our products have sustained serious damage in transit such that they were no longer usable. Although we have taken steps to improve our packaging and shipping containers, there is no guarantee our products will not become damaged or lost in transit in the future. If our products are damaged or lost in transit, it may result in a substantial delay in the fulfillment of our customer’s order and, depending on the type and extent of the damage, it may result in a substantial financial loss. If our products are not delivered in a timely fashion or are damaged or lost during the delivery process, our customers could become dissatisfied and cease using our products or our services, which would adversely affect our business, financial condition, results of operations, cash flows and prospects.
34
If we are unable to continue to hire and retain skilled personnel, we will have trouble developing and marketing our products and services.
Our success depends largely upon the continued service of our management and scientific staff and our ability to attract, retain and motivate highly skilled technical, scientific, management and marketing personnel, who deliver high-quality and timely services to our customers and keep pace with cutting-edge technologies and developments in biologics. We also face significant competition in the hiring and retention of such personnel from other companies, other providers of outsourced biologics services, research and academic institutions, government and other organizations who have superior funding and resources. The loss of key personnel or our inability to hire and retain skilled personnel could materially adversely affect the development of our products and services and our business, financial condition, results of operations, cash flows and prospects.
We may enter into additional distribution arrangements and marketing alliances for certain products and services and any failure to successfully identify and implement these arrangements on favorable terms, if at all, may impair our ability to effectively distribute and market our products.
We may pursue additional arrangements regarding the sales and marketing and distribution of one or more of our products and services and our future revenue may depend, in part, on our ability to enter into and maintain arrangements with other companies having sales, marketing and distribution capabilities and the ability of such companies to successfully market and sell any such products and services. Any failure to enter into such arrangements and marketing alliances on favorable terms, if at all, could delay or impair our ability to distribute or market our products and services and could increase our costs of distribution and marketing. Any use of distribution arrangements and marketing alliances to commercialize our products and services will subject us to a number of risks, including the following:
| • | we may be required to relinquish important rights to our products; |
| • | we may not be able to control the amount and timing of resources that our distributors or collaborators may devote to the distribution or marketing of our products; |
| • | our distributors or collaborators may experience financial difficulties; and |
| • | business combinations or significant changes in a collaborator’s business strategy may adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement. |
Our success depends on the market acceptance of our life science reagents. Our reagents may not achieve or maintain significant commercial market acceptance.
Our commercial success is dependent upon our ability to continue to successfully market and sell our life science reagents. Our ability to achieve and maintain commercial market acceptance of our products and services and provide customers access to our life science reagents will depend on a number of factors, including:
| • | our ability to increase awareness of the capabilities of our technology and solutions; |
| • | our customers’ willingness to adopt new products, services and technologies; |
| • | whether our products and services reliably provide advantages over legacy and other alternative technologies and are perceived by customers to be cost effective; |
| • | our ability to execute on our strategy to scale-up our CleanCap® technology to meet increasing demand and provide channels to access our CleanCap® technology and life science reagents; |
| • | the rate of adoption of our products and services by biopharmaceutical companies, academic institutions and others; |
| • | the relative reliability and robustness of our products and services as a whole and the components of our life science offerings, including, for example, CleanCap®, our assays for detecting host cell proteins and research products for labeling and detecting proteins; |
35
| • | our ability to develop new tools and solutions for customers; |
| • | whether competitors develop and commercialize products and services that provide comparable features and benefits at scale; |
| • | the impact of our investments in product innovation and commercial growth; |
| • | negative publicity regarding our or our competitors’ products resulting from defects or errors; and |
| • | our ability to further validate our technology through research and accompanying publications. |
We cannot assure you that we will be successful in addressing these criteria or other criteria that might affect the market acceptance of our products and services. If we are unsuccessful in achieving and maintaining market acceptance of our products and services, our business, financial condition, results of operations, cash flows and prospects could be adversely affected.
The market may not be receptive to our new products and services upon their introduction.
We expect a portion of our future revenue growth to come from introducing new nucleic acid products, including plasmid DNA. The commercial success of all of our products and services will depend upon their acceptance by the life science and biopharmaceutical industries. Some of the products and services that we are developing are based upon new technologies or approaches. As a result, there can be no assurance that these new products and services, even if successfully developed and introduced, will be accepted by customers. If customers do not adopt our new products, services and technologies, our results of operations may suffer and, as a result, the market price of our Class A common stock may decline.
It may be difficult for us to implement our strategies for revenue growth in light of competitive challenges.
We face significant competition across many of our product lines. In addition, consolidation trends in the pharmaceutical, biotechnology and diagnostics industries have served to create fewer customer accounts and to concentrate purchasing decisions for some customers, resulting in increased pricing pressure on us. Moreover, customers may believe that larger companies are better able to compete as sole source vendors, and therefore prefer to purchase from such businesses. Failure to anticipate and respond to competitors’ actions may impact our future revenue and profitability.
The estimates of market opportunity and forecasts of market growth included in this prospectus may prove to be inaccurate, and even if the market in which we compete achieves the forecasted growth, our business could fail to grow at similar rates, if at all.
Addressable market estimates and growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. These estimates and forecasts are based on a number of complex assumptions and third-party estimates and other business data, including assumptions and estimates relating to our ability to generate revenue from existing products and services and the development of new products and services. The estimates and forecasts in this prospectus relating to the size and expected growth of our markets may prove to be inaccurate. Even if the markets in which we compete meet the size estimates and growth forecasted in this prospectus, our business could fail to grow at the rate we anticipate, if at all.
Product liability lawsuits against us could cause us to incur substantial liabilities, limit sales of our existing products and limit commercialization of any products that we may develop.
Our business exposes us to the risk of product liability claims that are inherent in the development, production, distribution, and sale of biotechnology products. We face an inherent risk of product liability exposure related to the use of certain of our products in our customers’ human clinical trials and product liability lawsuits may allege that our products or services identified inaccurate or incomplete information or otherwise failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of or
36
inappropriate reliance upon, the information we provide in the ordinary course of our business activities. If any of our products harm people due to our negligence, willful misconduct, unlawful activities or material breach, or if we cannot successfully defend ourselves against claims that our products caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in the following, any of which could impact our business, financial condition, results of operations, cash flows and prospects:
| • | decreased demand for our products and any products that we may develop; |
| • | injury to our reputation; |
| • | costs to defend the related litigation; |
| • | loss of revenue; and |
| • | the inability to commercialize products that we may develop. |
We maintain product liability insurance, but this insurance is subject to deductibles, limits and exclusions and may not fully protect us from the financial impact of defending against product liability claims or the potential loss of revenue that may result. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future.
We are subject to stringent privacy laws, information security laws, regulations, policies and contractual obligations related to data privacy and security and changes in such laws, regulations, policies and contractual obligations could adversely affect our business, financial condition, results of operations, cash flows and prospects.
We are subject to data privacy and protection laws and regulations that apply to the collection, transmission, storage and use of proprietary information and personally-identifying information, which among other things, imposes certain requirements relating to the privacy, security and transmission of certain individually identifiable information.
Numerous other federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure and security of personal information. These laws continue to change and evolve and are increasing in breadth and impact. Failure to comply with any of these laws and regulations could result in enforcement action against us, including fines, imprisonment of company officials and public censure, claims for damages by affected individuals, damage to our reputation and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects. Additionally, if we are unable to properly protect the privacy and security of personal information, we could be found to have breached our contracts.
Many states in which we operate have laws that protect the privacy and security of personal information. For example, the California Consumer Privacy Act of 2018 (“CCPA”), which increases privacy rights for California residents and imposes obligations on companies that process their personal information, came into effect on January 1, 2020. Among other things, the CCPA requires covered companies to provide new disclosures to California consumers and provide such consumers new data protection and privacy rights, including the ability to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal information. This law and others like it are as yet untested and may subject us to increased regulatory scrutiny, litigation, and overall risk. State laws are changing rapidly and there is discussion in Congress of a new federal data protection and privacy law to which we would become subject, if it is enacted.
Various foreign countries in which we operate also have, or are developing, laws that govern the collection, use, disclosure, security and cross-border transmission of personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy
37
and data protection issues that have the potential to affect our business. For example, privacy requirements in the European Union (the “EU”) govern the transfer of personal information from the European Economic Area to the United States. In the EU and the United Kingdom, the collection and use of personal data is governed by the provisions of the General Data Protection Regulation (“GDPR”), in addition to other applicable laws and regulations. The GDPR came into effect in May 2018, repealing and replacing the European Union Data Protection Directive, and imposing revised data privacy and security requirements on companies in relation to the processing of personal data of EU and United Kingdom data subjects. The GDPR, together with national legislation, regulations and guidelines of EU member states and the United Kingdom governing the processing of personal data, impose strict obligations with respect to, and restrictions on, the collection, use, retention, protection, disclosure, transfer and processing of personal data. The GDPR authorizes fines for certain violations of up to 4% of a company’s total global annual turnover for the preceding financial year or €20 million, whichever is greater. Such fines are in addition to any civil litigation claims by data subjects. Brexit may also lead to further legislative and regulatory changes and increase our compliance costs. The United Kingdom has transposed the GDPR into domestic law, with a United Kingdom version of the GDPR taking effect in January 2021, after the end of the Brexit transitional period. This could have the result of exposing us to two parallel data privacy regimes in Europe, each of which potentially authorizes significant fines for certain violations. Other jurisdictions outside the EU are similarly introducing or enhancing privacy and data security laws, rules and regulations, which could increase our compliance costs and the risks associated with noncompliance. We cannot guarantee that we are, or will be, in compliance with all applicable international regulations as they are enforced now or as they evolve.
It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices and our efforts to comply with the evolving data protection rules may be unsuccessful. We must devote significant resources to understanding and complying with this changing landscape. Failure to comply with federal, state and international laws regarding privacy and security of personal information could expose us to penalties under such laws, orders requiring that we change our practices, claims for damages or other liabilities, regulatory investigations and enforcement action, litigation and significant costs for remediation, any of which could adversely affect our business. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
We may be unable to efficiently manage growth as a larger and more geographically diverse organization.
Our strategic acquisitions, the continued expansion of our commercial sales operations and our organic growth have increased the scope and complexity of our business. As a result, we will face challenges inherent in efficiently managing a more complex business with an increased number of employees over large geographic distances, including the need to implement appropriate systems, policies, benefits and compliance programs. Our inability to manage successfully the geographically more diverse and substantially larger combined organization could materially adversely affect our operating results.
Opportunistic acquisitions may pose risks and challenges.
We have completed six acquisitions and several investments since April 2016 and, going forward, we may opportunistically pursue strategic acquisitions. However, we may be unable to continue to identify or complete promising acquisitions for many reasons, including competition among buyers, the high valuations of businesses in our industry, the need for regulatory and other approvals and the availability of capital. There can be no assurance that we will engage in any additional acquisitions or that we will be able to do so on terms that will enable us to realize the anticipated benefits. In addition, acquisitions financed with borrowings could increase our leverage and interest expense, which could make us more vulnerable to business downturns.
38
Our internal computer systems, or those of our customers, collaborators or other contractors, have been and may in the future be subject to cyber-attacks or security breaches, which could result in a material disruption of our product development programs or otherwise adversely affect our business, financial condition, results of operations, cash flows and prospects.
Despite the implementation of security measures, our internal computer systems and those of our customers are vulnerable to damage from computer viruses and unauthorized access. Cyber-attacks are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. Cyber-attacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. Cyber-attacks also could include phishing attempts or e-mail fraud to cause unauthorized payments or information to be transmitted to an unintended recipient. A material cyber-attack or security breach could cause interruptions in our operations and could result in a material disruption of our business operations, damage to our reputation, financial condition, results of operations, cash flows and prospects.
In the ordinary course of our business, we collect and store sensitive data, including, among other things, personally identifiable information about our employees, intellectual property, and proprietary business information. Any cyber-attack or security breach that leads to unauthorized access, use or disclosure of personal or proprietary information could harm our reputation, cause us not to comply with federal and/or state breach notification laws and foreign law equivalents and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information. In addition, we could be subject to risks caused by misappropriation, misuse, leakage, falsification or intentional or accidental release or loss of information maintained in the information systems and networks of our company and our vendors, including personal information of our employees, and company and vendor confidential data. In addition, outside parties have previously attempted and may in the future attempt to penetrate our systems or those of our vendors or fraudulently induce our personnel or the personnel of our vendors to disclose sensitive information in order to gain access to our data and/or systems or make unauthorized payments to third parties. Like other companies, we have on occasion experienced, and will continue to experience, data security incidents involving access to company data, unauthorized payments and threats to our data and systems, including malicious codes and viruses, phishing, business email compromise attacks, or other cyber-attacks. The number and complexity of these threats continue to increase over time. If a material breach of our information technology systems or those of our vendors occurs, the market perception of the effectiveness of our security measures could be harmed and our reputation and credibility could be damaged.
We could be required to expend significant amounts of money and other resources to respond to these threats or breaches and to repair or replace information systems or networks and could suffer financial loss or the loss of valuable confidential information. In addition, we could be subject to regulatory actions and/or claims made by individuals and groups in private litigation involving privacy issues related to data collection and use practices and other data privacy laws and regulations, including claims for misuse or inappropriate disclosure of data, as well as unfair or deceptive practices. Although we develop and maintain systems and controls designed to prevent these events from occurring, and we have a process to identify and mitigate threats, the development and maintenance of these systems, controls and processes is costly and requires ongoing monitoring and updating as technologies change and efforts to overcome security measures become increasingly sophisticated. Moreover, despite our efforts, the possibility of these events occurring cannot be eliminated entirely and there can be no assurance that any measures we take will prevent cyber-attacks or security breaches that could adversely affect our business, financial condition, results of operations, cash flows and prospects.
We are subject to export and import control laws and regulations that could impair our ability to compete in international markets or subject us to liability if we violate such laws and regulations.
We are subject to U.S. export controls and sanctions regulations that restrict the shipment or provision of certain products and services to certain countries, governments and persons. While we take precautions to prevent our products and services from being exported in violation of these laws, we cannot guarantee that the
39
precautions we take will prevent violations of export control and sanctions laws. If we are found to be in violation of U.S. sanctions or export control laws, it could result in substantial fines and penalties for us and for the individuals working for us. We may also be adversely affected through other penalties, reputational harm, loss of access to certain markets, or otherwise. Complying with export control and sanctions regulations may be time consuming and may result in the delay or loss of sales opportunities or impose other costs. Any change in export or import regulations, economic sanctions or related legislation, or change in the countries, governments, persons or technologies targeted by such regulations, could result in our decreased ability to export or sell certain products and services to existing or potential customers in affected jurisdictions.
We are subject to risks related to Brexit.
On January 31, 2020, the United Kingdom (the “UK”) left the EU, which is commonly referred to as “Brexit.” Brexit creates an uncertain political and economic environment in the UK and potentially across other EU member states for the foreseeable future, including during any period while the terms of the future relationship between the UK and EU are being negotiated and such uncertainties could impair or limit our ability to transact business in the member EU states. Additionally, there also is a risk that other countries may decide to leave the EU.
Further, Brexit could adversely affect European and worldwide economic or market conditions and could contribute to instability in global financial markets, and the value of the Pound Sterling currency or other currencies, including the Euro. We are exposed to the economic, market and fiscal conditions in the UK and the EU and to changes in any of these conditions. Consequently, no assurance can be given as to the impact of Brexit, or continued uncertainty regarding it, and, in particular, no assurance can be given that our operating results, financial condition and prospects would not be adversely impacted by the result.
Changes in political, economic or governmental regulations may reduce demand for our products and services or increase our expenses.
We compete in many markets in which we and our customers must comply with federal, state, local and international regulations, such as environmental, health and safety and food and drug regulations. We develop, configure and market our products and services to meet customer needs created by those regulations. The U.S. and international healthcare industry is subject to changing political, economic and regulatory influences that could significantly affect the drug development process, research and development costs and the pricing and reimbursement for pharmaceutical products. Any significant change in regulations could have an adverse effect on both our customers’ business and our business, which could result in reduced demand for our products and services or increases in our expenses. For example, we provide products and services used for basic research, raw materials used by biopharmaceutical customers for further processing, and active pharmaceutical ingredients used for preclinical studies and clinical trials.
Changes in the FDA’s regulation of the drug discovery and development process may have a negative impact on the ability of our customers to conduct and fund clinical trials, which could have a material adverse effect on the demand for the products and services we provide these customers. Additionally, the U.S. government and governments worldwide have increased efforts to expand healthcare coverage while at the same time curtailing and better controlling the increasing costs of healthcare. If cost-containment efforts limit our customers’ profitability, they may decrease research and development spending, which could decrease the demand for our products and services and materially adversely affect our growth prospects. Any of these factors could harm our customers’ businesses, which, in turn, could materially adversely hurt our business, financial condition, results of operations, cash flows and prospects.
We are subject to financial, operating, legal and compliance risk associated with global operations.
We engage in business globally, with approximately 40%, 41% and 42% of our revenue for the years ended December 31, 2018 and 2019 and the nine months ended September 30, 2020, respectively, coming from outside
40
the U.S. In addition, one of our strategies is to expand geographically, both through distribution and through direct sales. This subjects us to a number of risks, including international economic, political, and labor conditions; currency fluctuations; tax laws (including U.S. taxes on income earned by foreign subsidiaries); increased financial accounting and reporting burdens and complexities; unexpected changes in, or impositions of, legislative or regulatory requirements; failure of laws to protect intellectual property rights adequately; inadequate local infrastructure and difficulties in managing and staffing international operations; delays resulting from difficulty in obtaining export licenses for certain technology; tariffs, quotas and other trade barriers and restrictions; transportation delays; operating in locations with a higher incidence of corruption and fraudulent business practices; and other factors beyond our control, including terrorism, war, natural disasters, climate change and diseases.
The application of laws and regulations implicating global transactions is often unclear and may at times conflict. Compliance with these laws and regulations may involve significant costs or require changes in our business practices that result in reduced revenue and profitability. Non-compliance could also result in fines, damages, criminal sanctions, prohibited business conduct, and damage to our reputation. We incur additional legal compliance costs associated with our global operations and could become subject to legal penalties in foreign countries if we do not comply with local laws and regulations, which may be substantially different from those in the U.S.
We may expand our operations in countries with developing economies, where it may be common to engage in business practices that are prohibited by anti-corruption and anti-bribery laws and regulations that apply to us, such as the U.S. Foreign Corrupt Practices Act (FCPA), the U.S. Travel Act, and the UK Bribery Act 2010, which prohibit improper payments or offers of payment to foreign governments and political parties by us for the purpose of obtaining or retaining business. Although we implement policies and procedures designed to ensure compliance with these laws, there can be no assurance that all of our employees, contractors, distributors and agents, including those based in foreign countries where practices which violate such U.S. laws may be customary, will comply with our internal policies. Any such non-compliance, even if prohibited by our internal policies, could have an adverse effect on our business and result in significant fines or penalties.
Our acquisitions expose us to risks that could adversely affect our business, and we may not achieve the anticipated benefits of acquisitions of businesses or technologies.
We have made in the past, and may make in the future, selected opportunistic acquisitions of complementary businesses, products, services or technologies. In April 2016, we acquired Vector Laboratories, Inc. (“Vector Laboratories”), which allowed our entry into the protein detection business. In September 2016, we acquired TriLink BioTechnologies, Incorporated (“TriLink BioTechnologies”), in December 2016 we acquired the assets of Solulink Incorporated (“Solulink”) and in December 2017 we acquired Glen Research Corporation (“Glen Research”), which together have formed our nucleic acid business and production capabilities. In October 2016 we acquired Cygnus Technologies, LLC (“Cygnus Technologies”) and in March 2020 we acquired MockV Solutions, Inc., which together constitute biologics safety testing business.
Any acquisition involves numerous risks, uncertainties and operational, financial, and managerial challenges, including the following, any of which could adversely affect our business, financial condition, results of operations, cash flows and prospects:
| • | difficulties in integrating new operations, systems, technologies, products, services and personnel of acquired businesses effectively; |
| • | problems maintaining uniform procedures, controls and policies with respect to our financial accounting systems; |
| • | lack of synergies or the inability to realize expected synergies and cost-savings, including enhanced revenue, technology, human resources, cost savings, operating efficiencies and other synergies; |
41
| • | difficulties in managing geographically dispersed operations, including risks associated with entering foreign markets in which we have no or limited prior experience; |
| • | underperformance of any acquired technology, product, or business relative to our expectations and the price we paid; |
| • | negative near-term impacts on financial results after an acquisition, including acquisition-related earnings charges; |
| • | the potential loss of key employees, customers, and strategic partners of acquired companies; |
| • | declining employee morale and retention issues affecting employees of businesses that we acquire, which may result from changes in compensation, or changes in management, reporting relationships, future prospects or the direction of the acquired business; |
| • | claims by terminated employees and shareholders of acquired companies or other third parties related to the transaction; |
| • | the assumption or incurrence of additional debt obligations or expenses, or use of substantial portions of our cash; |
| • | the issuance of equity or equity-linked securities to finance or as consideration for any acquisitions that dilute the ownership of our shareholders; |
| • | the issuance of equity securities to finance or as consideration for any acquisitions may not be an option if the price of our Class A common stock is low or volatile which could preclude us from completing any such acquisitions; |
| • | any collaboration, strategic alliance and licensing arrangement may require us to relinquish valuable rights to our technologies or products, or grant licenses on terms that are not favorable to us; |
| • | disruption of our ongoing operations and diversion of management’s attention and company resources from existing operations of the business; |
| • | inconsistencies in standards, controls, procedures, and policies; |
| • | the impairment of intangible assets as a result of technological advancements, or worse-than-expected performance of acquired companies; |
| • | assumption of, or exposure to, historical liabilities of the acquired business, including unknown contingent or similar liabilities that are difficult to identify or accurately quantify, litigation-related liabilities and regulatory compliance or accounting issues, and potential litigation or regulatory action arising from a proposed or completed acquisition; |
| • | the need to later divest acquired assets at a loss if an acquisition does not meet our expectations; and |
| • | risks associated with acquiring intellectual property, including potential disputes regarding acquired companies’ intellectual property. In addition, the successful integration of acquired businesses requires significant efforts and expense across all operational areas, including sales and marketing, research and development, manufacturing, finance, legal, and information technologies. |
There can be no assurance that any of the acquisitions we have made, or that we may make, will be successful or will be, or will remain, profitable. Our failure to successfully address the foregoing risks may prevent us from achieving the anticipated benefits from any past or future acquisition in a reasonable time frame, or at all.
Our results of operations could be negatively affected by potential fluctuations in foreign currency exchange rates.
We conduct a significant portion of our business in international markets. We are exposed to the risk of an increase or decrease in the value of certain foreign currencies relative to the U.S. dollar, which could decrease the
42
value of our revenue when measured in U.S. dollars. As a result, our results of operation may be influenced by the effects of future exchange rate fluctuations and such effects may have an adverse impact on the market price of our Class A common stock.
Our products could become subject to more onerous regulation by the FDA or other regulatory agencies in the future, which could increase our costs and delay or prevent commercialization of our products, thereby materially and adversely affecting our business, financial condition, results of operations, cash flows and prospects.
We make certain of our products available to customers as research-use-only (“RUO”) products. RUO products are regulated by the FDA as medical devices, and include in vitro diagnostic products in the laboratory research phase of development that are being shipped or delivered for an investigation that is not subject to the FDA’s investigational device exemption requirements. Although medical devices are subject to stringent FDA oversight, products that are intended for RUO and are labeled as RUO are exempt from compliance with most FDA requirements, including premarket clearance or approval, manufacturing requirements, and others. A product labeled RUO but which is actually intended for clinical diagnostic use may be viewed by the FDA as adulterated and misbranded under the FDCA, and subject to FDA enforcement action. The FDA has indicated that when determining the intended use of a product labeled RUO, the FDA will consider the totality of the circumstances surrounding distribution and use of the product, including how the product is marketed and to whom. The FDA could disagree with our assessment that our products are properly marketed as RUO, or could conclude that products labeled as RUO are actually intended for clinical diagnostic use, and could take enforcement action against us, including requiring us to stop distribution of our products until we are in compliance with applicable regulations, which would reduce our revenue, increase our costs and adversely affect our business, prospects, results of operations and financial condition. In the event that the FDA requires us to obtain marketing authorization of our RUO products in the future, there can be no assurance that the FDA will grant any clearance or approval requested by us in a timely manner, or at all.
Our raw material products are manufactured following the voluntary quality standards of ISO 9001:2015. Our GMP-grade raw material products follow ISO 9001:2015 standards, additional voluntary GMP quality standards and customer specific requirements. We believe these raw material products, including our GMP-grade raw material products, are exempt from compliance with the FDCA and the cGMP regulations of the FDA, as our products are further processed by our customers and we do not make claims related to their safety or effectiveness. We provide API products to customers for use in preclinical studies through and including clinical trials. Our API products are manufactured following the principles detailed in the International Council for Harmonisation (ICH) Q7, Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients (Section 19, APIs For Use in Clinical Trials) in order to comply with the applicable requirements of the FDCA, and the comparable GMP principles for Europe; European Community, Part II, Basic Requirements for Active Substances Used as Starting Materials (Section 19, APIs For Use in Clinical Trials). Manufacture of APIs for use in clinical trials is regulated under § 501(a)(2)(B) of the FDCA, but is not subject to the current GMP regulations in 21 CFR § 211 by operation of 21 CFR § 210. Our API products are provided to customers under customer contracts that outline quality standards and product specifications. As products advance through the clinical phases, requirements become more stringent and we work with customers to define and agree on requirements and risks associated with their product.
The FDA could disagree with our assessment that our products are exempt from current GMP regulations. In addition, the FDA could conclude that the raw material and API products we provide to our customers are actually subject to the pharmaceutical or drug quality-related regulations for manufacturing, processing, packing or holding of drugs or finished pharmaceuticals, and could take enforcement action against us, including requiring us to stop distribution of our products until we are in compliance with applicable regulations, which would reduce our revenue, increase our costs and adversely affect our business, prospects, results of operations and financial condition. In the event that the FDA requires us to comply with FDA regulations, for our raw material and API products in the future, including the FDA’s current GMP regulations, there can be no assurance that the FDA will find our operations are in compliance in a timely manner, or at all.
43
Our ability to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future tax payments is limited by provisions of the Internal Revenue Code, and it is possible that certain transactions or a combination of certain transactions may result in material additional limitations on our ability to use our net operating loss and tax credit carryforwards.
Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, contain rules that limit the ability of a company that undergoes an ownership change, which is generally any change in ownership of more than 50% of its stock over a three-year period, to utilize its net operating loss and tax credit carryforwards and certain built-in losses recognized in years after the ownership change. These rules generally operate by focusing on ownership changes involving stockholders owning directly or indirectly 5% or more of the stock of a company and any change in ownership arising from a new issuance of stock by the company. Generally, if an ownership change occurs, the yearly taxable income limitation on the use of net operating loss and tax credit carryforwards and certain built-in losses is equal to the product of the applicable long-term, tax-exempt rate and the value of the company’s stock immediately before the ownership change. As a result, following any such ownership change, we might be unable to offset our taxable income with losses, or our tax liability with credits, before such losses and credits expire, in which event we could incur larger federal and state income tax liabilities than we would have had we not experienced an ownership change.
Our activities are and will continue to be subject to extensive government regulation, which is expensive and time consuming.
We are subject to various local, state, federal, foreign and transnational laws and regulations, and, in the future, any changes to such laws and regulations could adversely affect us.
We provide products and services used for basic research, raw materials and life science reagents used by biopharmaceutical customers for further processing, assays for biologics safety testing and active pharmaceutical ingredients used for preclinical studies and clinical trials. The quality of our products and services is critical to researchers looking to develop novel vaccines and therapies and for biopharmaceutical customers who use our products as raw materials or who are engaged in preclinical studies and clinical trials. Biopharmaceutical customers are subject to extensive regulations by the FDA and similar regulatory authorities in other countries for conducting clinical trials and commercializing products for therapeutic or diagnostic use. This regulatory scrutiny results in our customers imposing rigorous quality requirements on us as their supplier through supplier qualification processes and customer contracts.
Additionally, regulatory authorities and our customers may conduct scheduled or unscheduled periodic inspections of our facilities to monitor our regulatory compliance or compliance with our quality agreements with our customers. There are significant risks at each stage of the regulatory scheme for our customers.
Regulatory agencies may in the future take action against us or our customers for failure to comply with applicable regulations governing clinical trials and the development and testing of therapeutic products. Failure by us or by our customers to comply with the requirements of these regulatory authorities, including without limitation, remediating any inspectional observations to the satisfaction of these regulatory authorities, could result in warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution, restrictions on our operations, civil or criminal sanctions, or withdrawal of existing or denial of pending approvals, including those relating to products or facilities. In addition, such a failure could expose us to contractual or product liability claims, contractual claims from our customers, including claims for reimbursement for lost or damaged active pharmaceutical ingredients, as well as ongoing remediation and increased compliance costs, any or all of which could be significant.
We are also subject to a variety of federal, state, local and international laws and regulations that govern, among other things, the importation and exportation of products, the handling, transportation and manufacture of substances that could be classified as hazardous, and our business practices in the U.S. and abroad such as anti-corruption and anti-competition laws. Any noncompliance by us with applicable laws and regulations or the
44
failure to maintain, renew or obtain necessary permits and licenses could result in criminal, civil and administrative penalties and could have an adverse effect on our results of operations.
We may be required to record a significant charge to earnings if our goodwill and other amortizable intangible assets, or other investments become impaired.
We are required under GAAP to test goodwill for impairment at least annually and to review our goodwill, amortizable intangible assets and other assets acquired through merger and acquisition activity for impairment when events or changes in circumstance indicate the carrying value may not be recoverable. Factors that could lead to impairment of goodwill, amortizable intangible assets and other assets acquired via acquisitions include significant adverse changes in the business climate and actual or projected operating results (affecting our company as a whole or affecting any particular segment) and declines in the financial condition of our business. We may be required in the future to record additional charges to earnings if our goodwill, amortizable intangible assets or other investments become impaired. Any such charge would adversely impact our consolidated financial results.
Changes in accounting principles and guidance could result in unfavorable accounting charges or effects.
We prepare our consolidated financial statements in accordance with GAAP. These principles are subject to interpretation by the SEC and various bodies formed to create and interpret appropriate accounting principles and guidance. A change in these principles or guidance, or in their interpretations, may have a material effect on our reported results, as well as our processes and related controls, and may retroactively affect previously reported results. For example, during February 2016, the Financial Accounting Standards Board issued ASU 2016-02, Leases (Topic 842). The updated standard requires the recognition of a liability for lease obligations and a corresponding right-of-use asset on the balance sheet, and disclosures of certain information regarding leasing arrangements. We are currently assessing the timing and impact of adopting the updated provisions.
Our revenue recognition and other factors may impact our financial results in any given period and make them difficult to predict.
Under accounting standards update No. 2014-09 (Topic 606), Revenue from Contracts with Customers, (“ASC 606”), we recognize revenue when our performance obligations have been satisfied in an amount that reflects the consideration that we expect to receive in exchange for those performance obligations. Our revenue includes revenue from the sale of manufactured products, including products that can be purchased out of a catalog and custom manufactured products, and services, including custom antibody and assay development contracts, antibody affinity extraction and stability and feasibility studies, as well as certain licensing and royalty arrangements. The majority of our contracts include only one performance obligation, namely the delivery of products, both custom and catalog, and services. We also recognize revenue from other contracts that may include a combination of products and services, the provision of solely services, or from license fee arrangements which may be associated with the delivery of product. Our application of ASC 606 with respect to the nature of future contractual arrangements could impact the forecasting of our revenue for future periods, as both the mix of products and services we will sell in a given period, as well as the size of contracts, is difficult to predict. We adopted the requirements of ASC 606, effective January 1, 2019 using the modified retrospective method. Under the modified retrospective method, this guidance is applied to those contracts that were not completed as of January 1, 2019, with no restatement of contracts that were commenced and completed within fiscal years prior to January 1, 2019, and the prior period comparable financial information continues to be presented under the guidance of ASC 605, Revenue Recognition (ASC 605).
Furthermore, the presentation of our financial results requires us to make estimates and assumptions that may affect revenue recognition. In some instances, we could reasonably use different estimates and assumptions, and changes in estimates may occur from period to period. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates—Revenue Recognition.”
45
Given the foregoing factors, comparing our revenue and operating results on a period-to-period basis may not be meaningful, and our past results may not be indicative of our future performance.
Fluctuations in our effective tax rate may adversely affect our results of operations and cash flows.
We are subject to a variety of tax liabilities, including federal, state, foreign and other taxes such as income, sales/use, payroll, withholding, and ad valorem taxes. Changes in tax laws or their interpretations could decrease our net income, the value of any tax loss carryforwards, the value of tax credits recorded on our balance sheet and our cash flows, and accordingly could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects. In addition, some of our tax liabilities are subject to periodic audits by the relevant taxing authority, which could increase our tax liabilities.
Our business is subject to a number of environmental risks.
Our manufacturing business involves the controlled use of hazardous materials and chemicals and is therefore subject to numerous environmental and safety laws and regulations and to periodic inspections for possible violations of these laws and regulations. In addition to these hazardous materials and chemicals, our facility in Burlingame, California also produces certain toxins for research use that can cause severe illness in humans. The costs of compliance with environmental and safety laws and regulations are significant. Any violations, even if inadvertent or accidental, of current or future environmental and safety laws or regulations and the cost of compliance with any resulting order or fine could adversely affect our operations.
Risks Related to Our Intellectual Property
If we are unable to obtain, maintain and enforce intellectual property protection for our current or future products, or if the scope of our intellectual property protection is not sufficiently broad, our ability to commercialize our products successfully and to compete effectively may be materially adversely affected.
Our success depends on our ability to obtain and maintain patent and other intellectual property protection in the United States and other countries with respect to our current and future proprietary products. We rely upon a combination of patents and trade secret protection to protect the intellectual property related to our technology, manufacturing processes, and products. Our commercial success depends in part on obtaining and maintaining patent and trade secret protection of our current and future products, if any, and the methods used to manufacture them, as well as successfully defending such patents and trade secrets against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell or importing our products is dependent upon the extent to which we have rights under valid and enforceable patents and other intellectual property that covers these activities.
The patent prosecution process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner or in all jurisdictions where protection may be commercially advantageous. It is also possible that we may fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. In addition, we or our collaborators may only pursue, obtain or maintain patent protection in a limited number of countries. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found. We may be unaware of prior art that could be used to invalidate or narrow the scope of an issued patent or prevent our pending patent applications from issuing as patents. Because patent applications in the United States, Europe and many other non-U.S. jurisdictions are typically not published until 18 months after filing, or in some cases not at all, because publications of discoveries in scientific literature lag behind actual discoveries, and because we cannot be certain that we or our licensors were the first to make the inventions claimed in any of our owned or any in-licensed issued patents or pending patent applications, or that we or our licensors were the first to file for protection of the inventions set forth in our patents or patent applications. As a result, we may not be able to obtain or maintain protection for certain inventions. Even if
46
patents do successfully issue, such patents may not adequately protect our intellectual property, provide exclusivity for our current or future products, prevent others from designing around our claims or otherwise provide us with a competitive advantage. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patents or whether any issued patents will be found invalid or unenforceable or will be threatened by third parties. In addition, third parties may challenge the validity, enforceability, ownership, inventorship or scope of any of our patents. Any successful challenge to any of our patents could deprive us of rights necessary for the successful commercialization of our current or future products and could impair or eliminate our ability to collect future revenue and royalties with respect to such products. If any of our patent applications with respect to our current or future products fail to result in issued patents, if their breadth or strength of protection is narrowed or threatened, or if they fail to provide meaningful exclusivity or competitive position, it could dissuade companies from collaborating with us or otherwise adversely affect our competitive position.
The patent positions of life science companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in life science patents has emerged to date in the United States. The standards applied by the United States Patent and Trademark Office (the “USPTO”) and foreign patent offices in granting patents are not always applied uniformly or predictably, and can change. Additionally, the laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property rights, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement, misappropriation, or other violation of our patents or other intellectual property, including the unauthorized reproduction of our manufacturing or other know-how or the marketing of competing products in violation of our intellectual property rights generally. Any of these outcomes could impair our ability to prevent competition from third parties, which may have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Further, the existence of issued patents does not guarantee our right to practice the patented technology or commercialize products covered by such a patent. Third parties may have or obtain rights to patents which they may use to prevent or attempt to prevent us from practicing our patented technology or commercializing our patented products. If any of these other parties are successful in obtaining valid and enforceable patents, and establishing our infringement of those patents, we could be prevented from selling our products unless we were able to obtain a license under such third-party patents, which may not be available on commercially reasonable terms or at all. In addition, third parties may seek approval to market their own products similar to or otherwise competitive with our products. In these circumstances, we may need to defend or assert our patents, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or agency of competent jurisdiction may find our patents invalid or unenforceable. Our competitors and other third parties may also be able to circumvent our patents by developing similar or alternative products in a non-infringing manner. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
In addition, competitors may use our technologies in jurisdictions where we have not obtained or are unable to adequately enforce patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States and Europe. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing with us. Proceedings to enforce our patent rights, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or held unenforceable, or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual
47
property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop, acquire or license.
Intellectual property that we own or in-license may be subject to a reservation of rights by one or more third parties. For example, one of our patents is co-owned with third parties and some of our patent rights in the future may be co-owned with third parties. If we are unable to obtain an exclusive license to any such third-party co-owners’ interest in such patent rights, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In addition, we may need the cooperation of any such co-owners of such patent rights in order to enforce such patent rights against third parties, and such cooperation may not be provided to us. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
Moreover, the research resulting in certain of our patents and technology was funded in part by the U.S. government. As a result, the U.S. government has certain rights to such patent rights and technology, which include march-in rights. When new technologies are developed with government funding, in order to secure ownership of such patent rights, the recipient of such funding is required to comply with certain government regulations, including timely disclosing the inventions claimed in such patent rights to the U.S. government and timely electing title to such inventions. Additionally, the U.S. government generally obtains certain rights in any resulting patents, including a nonexclusive license authorizing the government to use the invention or to have others use the invention on its behalf. Accordingly, we or our licensors have granted the U.S. government a nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States, the inventions described in the patents and patent applications relating to such inventions. If the U.S. government decides to exercise these rights, it is not required to engage us as its contractor in connection with doing so. The government’s rights may also permit it to disclose our confidential information to third parties and to exercise march-in rights to use or allow third parties to use such government-funded technology. The government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, or because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. industry. In addition, our rights in such inventions may be subject to certain requirements to manufacture products embodying such inventions in the United States. If we fail to comply with those requirements, we could lose our ownership of or other rights to any patents subject to such regulations. Any exercise by the government of any of the foregoing rights or by any third party of its reserved rights could have a material adverse effect on our competitive position, business, financial condition, results of operations, and prospects.
Furthermore, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after its effective filing date. Various extensions may be available, however, the life of a patent and the protection it affords is limited. Given the amount of time required for the development, testing, regulatory review and approval of new products, our patents protecting such candidates might expire before or shortly after such candidates are commercialized. If we encounter delays in obtaining regulatory approvals, the period of time during which we could market a product under patent protection could be further reduced. Even if patents covering our future products are obtained, once such patents expire, we may be vulnerable to competition from similar products. The launch of a similar version of one of our products would likely result in an immediate and substantial reduction in the demand for our product. For example, certain patents related to our SoluLINK products expired in 2020 and certain other patents related to such products are due to expire in 2021 and 2022. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
If we are unable to protect the confidentiality of our proprietary information, the value of our technology and products could be materially adversely affected.
We also may rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. To maintain the confidentiality of trade secrets and other proprietary
48
information, we enter into confidentiality agreements with our employees, consultants, contractors, collaborators, contract development and manufacturing organizations (“CDMOs”), contract research organizations (“CROs”) and others upon the commencement of their relationships with us. These agreements require that all confidential information developed by the individual or entity or made known to the individual or entity by us during the course of the individual’s or entity’s relationship with us be kept confidential and not disclosed to third parties. Our agreements with employees as well as our personnel policies also generally provide that any inventions conceived by the individual in the course of rendering services to us shall be our exclusive property or that we may obtain full rights to such inventions at our election. However, trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, collaborators, CDMOs, CROs and others may unintentionally or willfully disclose our information to competitors. We also face the risk that present or former employees could continue to hold rights to intellectual property used by us, demand the registration of intellectual property rights in their name, and seek payment of damages for our use of such intellectual property.
Enforcing a claim that a third party illegally obtained or is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. We may not have adequate remedies in the event of unauthorized use or disclosure of our trade secrets or other proprietary information in the case of a breach of any such agreements and our trade secrets and other proprietary information could be disclosed to third parties, including our competitors. Many of our partners also collaborate with our competitors and other third parties. The disclosure of our trade secrets to our competitors, or more broadly, would impair our competitive position and may materially harm our business, financial condition, results of operations, cash flows and prospects. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our rights, and failure to maintain trade secret protection could adversely affect our competitive business position. The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction. Courts outside the United States are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop substantially equivalent or superior knowledge, methods and know-how, and the existence of our own trade secrets affords no protection against such independent discovery.
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful and could result in a court or administrative body finding our patents to be invalid or unenforceable.
Even if the patent applications we own or license are issued, third parties may challenge or infringe upon our patents. To counter infringement, we may be required to file infringement claims, which can be expensive and time-consuming. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including novelty, non-obviousness (or inventive step), written description or enablement. In addition, patent validity challenges may, under certain circumstances, be based upon non-statutory obviousness-type double patenting, which, if successful, could result in a finding that the claims are invalid for obviousness-type double patenting or the loss of patent term if a terminal disclaimer is filed to obviate a finding of obviousness-type double patenting. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld information material to patentability from the USPTO, or made a misleading statement, during prosecution.
Third parties may raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation or cancellation of or amendment to our patents in such a way that they no longer cover our current or future products or provide any competitive advantage. The outcome following legal assertions of invalidity and unenforceability is unpredictable. If a third party were to prevail on a legal assertion of invalidity or unenforceability, we could lose part or all of the patent protection on one or more of our current or future products, which could result in our competitors and other third
49
parties using our technology to compete with us. Such a loss of patent protection could have a material adverse impact on our business, financial condition, results of operations, cash flows and prospects.
Interference proceedings, or other similar enforcement and revocation proceedings, provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, infringement, misappropriation or other violation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
In an infringement proceeding, even one initiated by us, there is a risk that a court will decide that our patents are not valid and that we do not have the right to stop the other party from using the inventions they describe. There is also the risk that, even if the validity of such patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe our rights to these patents.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations. In addition, patent holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us, especially as we gain greater visibility and market exposure as a public company.
An adverse outcome in a litigation or proceeding involving our patents could limit our ability to assert our patents against competitors, affect our ability to receive royalties or other licensing consideration from our licensees, and may curtail or preclude our ability to exclude third parties from making, using and selling similar or competitive products. Any of these occurrences could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
If we are sued for infringing, misappropriating, or otherwise violating intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing our current or future products.
Our products may infringe on, or be accused of infringing on, one or more claims of an issued patent or may fall within the scope of one or more claims in a published patent application that may be subsequently issued and to which we do not hold a license or other rights.
Because patent applications in the United States and many foreign jurisdictions are typically not published until 18 months after filing, or in some cases not at all, and publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our issued patents or our pending applications, or that we were the first to invent the technology. Others, including our competitors, may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our patent applications or patents, which could further require us to obtain rights to issued patents by others covering such technologies. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful if, unbeknownst to us, the other party had independently arrived at the same or similar invention prior to our own invention, resulting in a loss of our U.S. patent position with respect to such inventions.
50
Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our current or future products or the use of our current or future products. After issuance, the scope of patent claims remains subject to construction based on interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. These third parties could bring claims against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages.
The life sciences industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Third parties have, and may in the future have, U.S. and non-U.S. issued patents and pending patent applications that may cover our current or future products. Such a third party may claim that we or our manufacturing or commercialization partners are using inventions covered by the third party’s patent rights and may go to court or a tribunal to stop us from engaging in our normal operations and activities, including making or selling our current or future products. In the event that any of these patent rights were asserted against us, we believe that we have defenses against any such action, including that such patents would not be infringed by our current or future products and/or that such patents are not valid. However, if any such patent rights were to be asserted against us and our defenses to such assertion were unsuccessful, unless we obtain a license to such patents, we could be liable for damages, which could be significant and include treble damages and attorneys’ fees if we are found to willfully infringe such patents, and we could be precluded from commercializing any future products that were ultimately held to infringe such patents, any of which could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
If we are found to infringe the patent rights of a third party, or in order to avoid potential claims, we or our collaborators may choose or be required to seek a license from a third party and be required to pay license fees or royalties or both. These licenses may not be available on reasonable terms, or at all. In particular, any of our competitors that control intellectual property that we are found to infringe may be unwilling to provide us a license under any terms. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. Further, if a patent infringement suit is brought against us or our third-party service providers and if we are unable to successfully obtain rights to required third-party intellectual property, we may be required to expend significant time and resources to redesign our current or future products, or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis, and may delay or require us to abandon our development, manufacturing or sales activities relating to our current or future products. A finding of infringement could prevent us from commercializing our future products or force us to cease some of our business operations, which could harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
51
Intellectual property litigation and other proceedings could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, intellectual property litigation or other legal proceedings relating to our, our licensors’ or other third parties’ intellectual property claims may cause us to incur significant expenses and could distract our personnel from their normal responsibilities. Patent litigation and other proceedings may also absorb significant management time. If not resolved in our favor, litigation may require us to pay any portion of our opponents’ legal fees. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Our competitors or other third parties may be able to sustain the cost of such litigation and proceedings more effectively than we can because of their substantially greater resources. Uncertainties resulting from our participation in patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Furthermore, because of the substantial amount of discovery required in certain jurisdictions in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, the perceived value of our current or future products or intellectual property could be diminished. Accordingly, the market price of our Class A common stock may decline. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our business, financial condition, results of operations, and prospects.
If we fail to comply with our obligations under any license agreements, disagree over contract interpretation, or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are necessary to our business.
We rely, in part, on intellectual property and technology which we have in-licensed. We may also need to obtain additional licenses in the future to advance our research or allow commercialization of our future products and it is possible that we may be unable to do so at a reasonable cost or on reasonable terms, if at all. Moreover, such licenses may not provide exclusive rights to use such intellectual property and technology in all relevant fields of use and in all territories in which we may wish to develop or commercialize our future products.
In addition, our existing license agreements impose, and any future license agreements we enter into may impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement or other obligations on us. Our license agreements, and any future license agreement we enter into, may also impose restrictions on our ability to license certain of our intellectual property to third parties or to develop or commercialize certain current or future products or technologies. In spite of our best efforts, our counterparties may conclude that we have breached our obligations under our agreements, or that we have used the intellectual property licensed to us in an unauthorized manner, in which case, we may be required to pay damages and the counterparty may have the right to terminate the agreement. Any of the foregoing could result in us being unable to develop, manufacture and sell products that are covered by the licensed intellectual property or technology, or enable a competitor to gain access to the licensed intellectual property or technology.
We might not have the necessary rights or the financial resources to develop, manufacture or market our current or future products without the rights granted under our license agreements, and the loss of sales or potential sales in current or future products covered by such license agreements could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Disputes may arise regarding intellectual property subject to license agreements, including:
| • | the scope of rights granted under the license agreement and other interpretation related issues; |
52
| • | the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the license agreement; |
| • | the sublicensing of patent and other rights under our collaborative development relationships; |
| • | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| • | our financial obligations under the license agreement; |
| • | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| • | the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology to or from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected future products.
In some cases, we may not have primary control over prosecution, maintenance, enforcement and defense of patents and patent applications that we have in-licensed from third parties, and instead we rely on our licensors for these activities. We cannot be certain that such activities have been or will be conducted in compliance with applicable laws and regulations or in a manner consistent with the best interests of our business. If we do undertake any enforcement of our in-licensed patents or defense of any claims asserting the invalidity of such patents, such actions may be subject to the cooperation of our licensors or other third parties. If our licensors or other third parties fail to prosecute, maintain, enforce and defend intellectual property licensed to us, or lose their own rights to such intellectual property, the rights we have licensed may be impaired or eliminated and our ability to develop and commercialize any of our products that are subject to such rights could be adversely affected.
In-licensing or acquisition of third-party intellectual property is a competitive area and a number of more established companies are also pursuing strategies to in-license or acquire third-party intellectual property rights that we may consider attractive or necessary for our business. These companies may have a competitive advantage over us due to their size, cash resources and greater capabilities with respect to clinical development and commercialization. Furthermore, companies that perceive us as a competitor may be unwilling to assign or license rights to us. If we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have on reasonable terms or at all, we may have to abandon development of the relevant program or current or future product and our business, financial condition, results of operations, cash flows and prospects could suffer.
Changes to the patent law in the United States and other jurisdictions could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, thereby impairing our ability to protect our technologies and current or future products.
As is the case with other life sciences companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the life sciences industry involves both technological and legal complexity and is therefore costly, time consuming and inherently uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents.
53
For example, the Leahy-Smith America Invents Act (the “America Invents Act”), was signed into law on September 16, 2011, and many of the substantive changes became effective on March 16, 2013. The America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Specifically, the America Invents Act reforms United States patent law in part by changing the U.S. patent system from a “first to invent” system to a “first inventor to file” system. Under a “first inventor to file” system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor was the first to invent the invention. This will require us to be cognizant going forward of the time from invention to filing of a patent application and be diligent in filing patent applications. Circumstances may arise that could prevent us from promptly filing patent applications on our inventions and allow third parties to file patents claiming our inventions before we are able to do so. The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by the USPTO administered post grant proceedings, including reexamination proceedings, inter partes review, post grant review and derivation proceedings. These adversarial proceedings at the USPTO review patent claims without the presumption of validity afforded to U.S. patents in lawsuits in U.S. federal courts, and use a lower burden of proof than used in litigation in U.S. federal courts. Therefore, it is generally considered easier for a competitor or third party to have a U.S. patent invalidated in a USPTO post-grant review or inter partes review proceeding than in a litigation in a U.S. federal court.
In addition, the patent positions of companies in the life sciences industry are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways. In addition, the complexity and uncertainty of European patent laws have also increased in recent years. Complying with these laws and regulations could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and patent applications will be due to be paid to the USPTO and various government patent agencies outside the United States over the lifetime of our patents and patent applications and any patent rights we may own or license in the future. Additionally, the USPTO and various government patent agencies outside the United States require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In certain cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with rules applicable to the particular jurisdiction. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we or our licensors fail to maintain the patents and patent applications covering or otherwise protecting our current or future products, it could have a material adverse effect on our business. In addition, to the extent that we have responsibility for taking any action related to the prosecution or maintenance of patents or patent applications in-licensed from a third party, any failure on our part to maintain the in-licensed intellectual property could jeopardize our rights under the relevant license and may have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
54
We may be subject to claims by third parties asserting that our employees, consultants, independent contractors or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property and proprietary technology.
Many of our employees were previously employed at universities or other life science, biotechnology or pharmaceutical companies, including our competitors or potential competitors. We try to ensure that our employees do not use the proprietary information or know-how of others in their work for us. We may, however, be subject to claims that we or these employees have inadvertently or otherwise used or disclosed intellectual property, trade secrets or other proprietary information of any such employee’s former employer or that patents and applications we have filed to protect inventions of these individuals, even those related to one or more of our current or future products, are rightfully owned by their former or concurrent employer. Litigation may be necessary to defend against these claims. Even if we are successful in defending ourselves, such litigation could result in substantial costs to us or be distracting to our management. If we fail to defend any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on an exclusive basis or on commercially reasonable terms or at all.
In addition, while we typically require our employees, consultants and independent contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, or such agreements may be breached or alleged to be ineffective, and the assignment may not be self-executing, which may result in claims by or against us related to the ownership of such intellectual property or may result in such intellectual property becoming assigned to third parties. If we fail in enforcing or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
We may not be able to protect our intellectual property and proprietary rights throughout the world.
Filing, prosecuting, and defending patents on current or future products in all countries throughout the world would be prohibitively expensive, and the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Third parties may use our technologies in jurisdictions where we have not obtained or are unable to adequately enforce patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but enforcement is not as strong as that in the United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our intellectual property and proprietary rights generally. Proceedings to enforce our intellectual property and proprietary rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to
55
enforce our intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, cash flows and prospects may be adversely affected.
We rely on confidentiality agreements that, if breached, may be difficult to enforce and could have a material adverse effect on our business and competitive position.
Our policy is to enter agreements relating to the non-disclosure and non-use of confidential information with third parties, including our contractors, consultants, advisors and research collaborators, as well as agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them. However, these agreements can be difficult and costly to enforce. Moreover, to the extent that our contractors, consultants, advisors and research collaborators apply or independently develop intellectual property in connection with any of our projects, disputes may arise as to the proprietary rights to the intellectual property. If a dispute arises, a court may determine that the right belongs to a third party, and enforcement of our rights can be costly and unpredictable. In addition, we rely on trade secrets and proprietary know-how that we seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
| • | these agreements may be breached; |
| • | these agreements may not provide adequate remedies for the applicable type of breach; or |
| • | our trade secrets or proprietary know-how will otherwise become known. |
Any breach of our confidentiality agreements or our failure to effectively enforce such agreements would have a material adverse effect on our business and competitive position.
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest and our business, financial condition, results of operations, cash flows and prospects may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names or marks which we need for name recognition by potential partners or customers in our markets of interest. During trademark registration proceedings, we may receive rejections. Although we would be given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business, financial condition, results of operations, cash flows and prospects may be adversely affected.
56
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our proprietary and intellectual property rights is uncertain because such rights offer only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| • | others may be able to develop products that are similar to, or better than, our current or future products in a way that is not covered by the claims of the patents we license or may own currently or in the future; |
| • | we, or our licensing partners or current or future collaborators, might not have been the first to make the inventions covered by issued patents or pending patent applications that we license or may own currently or in the future; |
| • | we, or our licensing partners or current or future collaborators, might not have been the first to file patent applications for certain of our or their inventions; |
| • | our pending owned or in-licensed patent applications may not lead to issued patents; |
| • | we may choose not to file a patent for certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property; |
| • | our competitors or other third parties might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| • | it is possible that there are prior public disclosures that could invalidate our or our licensors’ patents; |
| • | the patents of third parties or pending or future applications of third parties, if issued, may have an adverse effect on our business; |
| • | any patents that we obtain may not provide us with any competitive advantages or may ultimately be found not to be owned by us, invalid or unenforceable; or |
| • | we may not develop additional proprietary technologies that are patentable. |
Should any of these events occur, they could significantly harm our business, financial conditions, results of operations, cash flows and prospects.
Risks Related to Our Indebtedness
Our existing indebtedness could adversely affect our business and growth prospects.
As of September 30, 2020, we had total current and long-term indebtedness outstanding of approximately $351.7 million, including term loans of $345.0 million, $15.0 million in borrowings under the Revolving Credit Facility (as defined herein) and unamortized debt issuance costs of $8.3 million. Our indebtedness, or any additional indebtedness we may incur, could require us to divert funds identified for other purposes for debt service and impair our liquidity position. If we cannot generate sufficient cash flow from operations to service our debt, we may need to refinance our debt, dispose of assets or issue equity to obtain necessary funds. We do not know whether we will be able to take any of these actions on a timely basis, on terms satisfactory to us or at all.
Our indebtedness, the cash flow needed to satisfy our debt and the covenants contained in the New Credit Agreement have important consequences, including:
| • | limiting funds otherwise available for financing our capital expenditures by requiring us to dedicate a portion of our cash flows from operations to the repayment of debt and the interest on this debt; |
| • | limiting our ability to incur or prepay existing indebtedness, pay dividends or distributions, dispose of assets, engage in mergers and consolidations, make acquisitions or other investments and make changes in the nature of the business, among other things; |
57
| • | making us more vulnerable to rising interest rates, as certain of our borrowings, including borrowings under the New Credit Agreement, bear variable rates of interest; and |
| • | making us more vulnerable in the event of a downturn in our business. |
Our level of indebtedness may place us at a competitive disadvantage to our competitors that are not as highly leveraged. Fluctuations in interest rates can increase borrowing costs. Increases in interest rates may directly impact the amount of interest we are required to pay and reduce earnings accordingly. In addition, tax laws, including the disallowance or deferral of tax deductions for interest paid on outstanding indebtedness, could have an adverse effect on our liquidity and our business, financial condition, results of operations, cash flows and prospects. Further, our New Credit Agreement contains customary affirmative and negative covenants and certain restrictions on operations that could impose operating and financial limitations and restrictions on us, including restrictions on our ability to enter into particular transactions and to engage in other actions that we may believe are advisable or necessary for our business.
We expect to use cash flow from operations to meet current and future financial obligations, including funding our operations, debt service requirements and capital expenditures. The ability to make these payments depends on our financial and operating performance, which is subject to prevailing economic, industry and competitive conditions and to certain financial, business, economic and other factors beyond our control.
Despite current indebtedness levels, we may incur substantially more indebtedness, which could further exacerbate the risks associated with our substantial indebtedness.
We may incur significant additional indebtedness in the future. We may also consider investments in joint ventures or acquisitions, which may increase our indebtedness. If new debt is added to our current indebtedness levels, the related risks that we face could intensify.
Variable rate indebtedness that we have incurred or may in the future incur will subject us to interest rate risk, which could cause our debt service obligations to increase significantly.
Certain of our borrowings, including certain borrowings under our New Credit Agreement, bear variable rates of interest. An increase in interest rates would increase our debt service obligations, which would have a negative impact on our net income and cash flows, including cash available for servicing our indebtedness.
The phase-out of the London Interbank Offered Rate (“LIBOR”), or the replacement of LIBOR with a different reference rate, may adversely affect interest rates.
Borrowings under our New Credit Agreement bear interest at rates determined using LIBOR as the reference rate. On July 27, 2017, the Financial Conduct Authority (the authority that regulates LIBOR) announced that it would phase out LIBOR by the end of 2021. It is unclear whether new methods of calculating LIBOR will be established such that it continues to exist after 2021, or if alternative rates or benchmarks will be adopted, and currently it appears highly likely that LIBOR will be discontinued or substantially modified by 2021. If LIBOR is unavailable, we may be required to pay interest on borrowings based on historical LIBOR, which may be higher than market rates prevailing at such time. Changes in the method of calculating LIBOR, or the replacement of LIBOR with an alternative rate or benchmark, may adversely affect interest rates and result in higher borrowing costs. This could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects. We cannot predict the effect of the potential changes to LIBOR or the establishment and use of alternative rates or benchmarks. Furthermore, we may need to renegotiate our New Credit Agreement or incur other indebtedness, and changes in the method of calculating LIBOR, or the use of an alternative rate or benchmark, may negatively impact the terms of such indebtedness.
58
We may not be able to generate sufficient cash flow to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under such indebtedness, which may not be successful.
Our ability to make scheduled payments or to refinance outstanding debt obligations depends on our financial and operating performance, which will be affected by prevailing economic, industry and competitive conditions and by financial, business and other factors beyond our control. We may not be able to maintain a sufficient level of cash flow from operating activities to permit us to pay the principal, premium, if any, and interest on our indebtedness. Any failure to make payments of interest and principal on our outstanding indebtedness on a timely basis would likely result in a reduction of our creditworthiness, which would also harm our ability to incur additional indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures and acquisitions, sell assets, seek additional capital or seek to restructure or refinance our indebtedness. Any refinancing of our indebtedness could be at higher interest rates and may require us to comply with more onerous covenants. Refinancings may not be successful and may not permit us to meet our scheduled debt service obligations. In the absence of such cash flows and resources, we could face substantial liquidity problems and might be required to sell material assets or operations to attempt to meet our debt service obligations. The financing documents governing our New Credit Agreement include certain restrictions on our ability to conduct asset sales and/or use the proceeds from asset sales for certain purposes. We may not be able to consummate these asset sales to raise capital or sell assets at prices and on terms that we believe are fair and any proceeds that we do receive may not be adequate to meet any debt service obligations then due. If we cannot meet our debt service obligations, the holders of our indebtedness may accelerate such indebtedness and, to the extent such indebtedness is secured, foreclose on our assets. In such an event, we may not have sufficient assets to repay all of our indebtedness.
The terms of the financing documents governing our New Credit Agreement restrict our current and future operations, particularly our ability to respond to changes or to take certain actions.
The financing documents governing our New Credit Agreement contain a number of restrictive covenants that impose significant operating and financial restrictions on us and may limit our ability to engage in acts that may be in our long-term best interests, including restrictions on our ability to:
| • | incur additional indebtedness; |
| • | incur liens; |
| • | merge, dissolve, liquidate, amalgamate, consolidate or sell all or substantially all of our assets; |
| • | declare or pay certain dividends, payments or distribution or repurchase or redeem certain capital stock; |
| • | permit our subsidiaries to enter into agreements restricting their ability to pay dividends, make loans, incur liens and sell assets; and |
| • | make certain investments. |
These restrictions could limit, potentially significantly, our operational flexibility and affect our ability to finance our future operations or capital needs or to execute our business strategy.
We may be unable to refinance our indebtedness.
We may need to refinance all or a portion of our indebtedness before maturity. We cannot assure you that we will be able to refinance any of our indebtedness on commercially reasonable terms or at all. There can be no assurance that we will be able to obtain sufficient funds to enable us to repay or refinance our debt obligations on commercially reasonable terms, or at all.
59
Our failure to raise additional capital or generate cash flows necessary to expand our operations and invest in new technologies in the future could reduce our ability to compete successfully and harm our competitive position and results of operations.
We may need to raise additional funds, and we may not be able to obtain additional debt or equity financing on favorable terms or at all. If we raise additional equity financing, our security holders may experience significant dilution of their ownership interests. If we engage in additional debt financing, we may be required to accept terms that restrict our ability to incur additional indebtedness, force us to maintain specified liquidity or other ratios or restrict our ability to pay dividends or make acquisitions. If we need additional capital and cannot raise it on acceptable terms, or at all, we may not be able to, among other things:
| • | develop and enhance our product offerings; |
| • | continue to expand our organization; |
| • | hire, train and retain employees; |
| • | respond to competitive pressures or unanticipated working capital requirements; or |
| • | pursue acquisition opportunities. |
In addition, if we issue additional equity to raise capital, your interest in us will be diluted.
Risks Related to Our Organizational Structure
Our principal asset is our interest in Topco LLC, and, accordingly, we depend on distributions from Topco LLC to pay our taxes and expenses, including payments under the Tax Receivable Agreement. Topco LLC’s ability to make such distributions may be subject to various limitations and restrictions.
We are a holding company and have no material assets other than our ownership of equity interests in Topco LLC. As such, we have no independent means of generating revenue or cash flow, and our ability to pay our taxes, satisfy our obligations under the Tax Receivable Agreement and pay operating expenses or declare and pay dividends, if any, in the future depends on the financial results and cash flows of Topco LLC and its subsidiaries and distributions we receive from Topco LLC. There can be no assurance that Topco LLC and its subsidiaries will generate sufficient cash flow to distribute funds to us or that applicable state law and contractual restrictions, including negative covenants in debt instruments of Topco LLC and its subsidiaries, will permit such distributions.
Topco LLC is treated as a partnership for U.S. federal income tax purposes and, as such, is not subject to any entity-level U.S. federal income tax. Certain wholly-owned subsidiaries of Topco LLC are taxed as corporations for U.S. federal and most applicable state, local income tax and foreign tax purposes. For U.S. federal income tax purposes, taxable income of Topco LLC is allocated to the LLC Unitholders, including us. Accordingly, we incur income taxes on our distributive share of any net taxable income of Topco LLC. Under the terms of the LLC Operating Agreement, Topco LLC is obligated to make tax distributions to LLC Unitholders, including us. In addition to tax and dividend payments, we also incur expenses related to our operations, including obligations to make payments under the Tax Receivable Agreement. Due to the uncertainty of various factors, we cannot estimate the likely tax benefits we may realize as a result of our purchase of LLC Units and LLC Unit exchanges, and the resulting amounts we are likely to pay out to LLC Unitholders pursuant to the Tax Receivable Agreement; however, we estimate that such payments may be substantial. Under the LLC Operating Agreement, tax distributions shall be made on a pro rata basis among the LLC Unitholders, and will be calculated without regard to any applicable basis adjustment under Section 743(b) of the Code.
We intend to cause Topco LLC to make cash distributions to the owners of LLC Units in amounts sufficient to (1) fund all or part of their tax obligations in respect of taxable income allocated to them and (2) cover our operating expenses, including payments under the Tax Receivable Agreement.
60
However, Topco LLC’s ability to make such distributions may be subject to various limitations and restrictions, such as restrictions on distributions that would violate either any contract or agreement to which Topco LLC or its subsidiaries is then a party, including debt agreements, or any applicable law, or that would have the effect of rendering Topco LLC or its subsidiaries insolvent. In addition, recently enacted legislation that is effective for taxable years beginning after December 31, 2017 may impute liability for adjustments to a partnership’s tax return on the partnership itself in certain circumstances, absent an election to the contrary. Topco LLC may be subject to material liabilities pursuant to this legislation and related guidance if, for example, its calculations of taxable income are incorrect. If we do not have sufficient funds to pay tax or other liabilities or to fund our operations, we may have to borrow funds, which could materially adversely affect our liquidity and financial condition and subject us to various restrictions imposed by any such lenders. To the extent that we are unable to make payments under the Tax Receivable Agreement, such payments generally will be deferred and will accrue interest until paid. Nonpayment for a specified period, however, may constitute a breach of a material obligation under the Tax Receivable Agreement and therefore accelerate payments due under the Tax Receivable Agreement, unless, generally, such nonpayment is due to a lack of sufficient funds. See “—Risks Related to Our Class A Common Stock and This Offering,” “Dividend Policy,” “Organizational Structure—Tax Receivable Agreement” and “Organizational Structure—Amended and Restated Operating Agreement of Topco LLC.”
Conflicts of interest could arise between our shareholders and MLSH 1, which may impede business decisions that could benefit our shareholders.
MLSH 1, which is controlled by GTCR and which will be the only holder of LLC Units other than us upon consummation of this offering, has the right to consent to certain amendments to the LLC Operating Agreement, as well as to certain other matters. MLSH 1 may exercise these voting rights in a manner that conflicts with the interests of our shareholders. Circumstances may arise in the future when the interests of MLSH 1 conflict with the interests of our shareholders. As we control Topco LLC, we have certain obligations to MLSH 1 as an LLC Unitholder that may conflict with fiduciary duties our officers and directors owe to our shareholders. These conflicts may result in decisions that are not in the best interests of shareholders.
The Tax Receivable Agreement requires us to make cash payments to MLSH 1 and MLSH 2 in respect of certain tax benefits to which we may become entitled, and we expect that the payments we will be required to make will be substantial.
In connection with the consummation of this offering, we will enter into a Tax Receivable Agreement with MLSH 1 and MLSH 2. Pursuant to the Tax Receivable Agreement, we will be required to make cash payments to MLSH 1 and MLSH 2, collectively, equal to 85% of the tax benefits, if any, that we actually realize, or, in some circumstances, are deemed to realize, as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement. Due to the uncertainty of various factors, we cannot precisely quantify the likely tax benefits we will realize as a result of the purchase of LLC Units and LLC Unit exchanges, and the resulting amounts we are likely to pay out to MLSH 1 and MLSH 2 pursuant to the Tax Receivable Agreement; however, we estimate that such payments may be substantial. See “Organizational Structure—Tax Receivable Agreement.” Payments under the Tax Receivable Agreement will be based on the tax reporting positions that we determine, which tax reporting positions will be based on the advice of our tax advisors. Any payments made by us to MLSH 1 and MLSH 2 under the Tax Receivable Agreement will generally reduce the amount of overall cash flow that might have otherwise been available to us. To the extent that we are unable to make payments under the Tax Receivable Agreement, such payments generally will be deferred and will accrue interest until paid. Nonpayment for a specified period, however, may constitute a breach of a material obligation under the Tax Receivable Agreement and therefore accelerate payments due under the Tax Receivable Agreement, unless, generally, such nonpayment is due to a lack of sufficient funds. Furthermore, our future obligation to make payments under the Tax Receivable Agreement could make us a less attractive
61
target for an acquisition, particularly in the case of an acquirer that cannot use some or all of the tax benefits that may be deemed realized under the Tax Receivable Agreement. The payments under the Tax Receivable Agreement are also not conditioned upon MLSH 1 maintaining a continued ownership interest in Topco LLC. See “Organizational Structure—Tax Receivable Agreement.”
The actual amount and timing of any payments under the Tax Receivable Agreement will vary depending upon a number of factors, including the timing of exchanges by MLSH 1, the amount of gain recognized by MLSH 1, the amount and timing of the taxable income we generate in the future and the federal tax rates then applicable.
The amounts that we may be required to pay to MLSH 1 and MLSH 2 under the Tax Receivable Agreement may be accelerated in certain circumstances and may also significantly exceed the actual tax benefits that we ultimately realize.
The Tax Receivable Agreement provides that if (1) certain mergers, asset sales, other forms of business combination or other changes of control were to occur, (2) we breach any of our material obligations under the Tax Receivable Agreement or (3) at any time, we elect an early termination of the Tax Receivable Agreement, then the Tax Receivable Agreement will terminate and our obligations, or our successor’s obligations, to make payments under the Tax Receivable Agreement would accelerate and become immediately due and payable. The amount due and payable in that circumstance is based on certain assumptions, including an assumption that we would have sufficient taxable income to fully utilize all potential future tax benefits that are subject to the Tax Receivable Agreement. See “Organizational Structure—Tax Receivable Agreement.” We may need to incur debt to finance payments under the Tax Receivable Agreement to the extent our cash resources are insufficient to meet our obligations under the Tax Receivable Agreement as a result of timing discrepancies or otherwise.
As a result of a change in control, material breach or our election to terminate the Tax Receivable Agreement early, (1) we could be required to make cash payments to MLSH 1 and MLSH 2 that are greater than the specified percentage of the actual benefits we ultimately realize in respect of the tax benefits that are subject to the Tax Receivable Agreement and (2) we would be required to make an immediate cash payment equal to the anticipated future tax benefits that are the subject of the Tax Receivable Agreement discounted in accordance with the Tax Receivable Agreement, which payment may be made significantly in advance of the actual realization, if any, of such future tax benefits. In these situations, our obligations under the Tax Receivable Agreement could have a substantial negative impact on our liquidity and could have the effect of delaying, deferring or preventing certain mergers, asset sales, other forms of business combination, or other changes of control. There can be no assurance that we will be able to finance our obligations under the Tax Receivable Agreement.
Our organizational structure, including the Tax Receivable Agreement, confers certain benefits upon MLSH 1 and MLSH 2 that will not benefit the other common shareholders to the same extent as they will benefit MLSH 1 and MLSH 2.
Our organizational structure, including the Tax Receivable Agreement, confers certain benefits upon MLSH 1, as the only other LLC Unitholder, and MLSH 2 that will not benefit the other holders of our common stock to the same extent. We will enter into a Tax Receivable Agreement with MLSH 1 and MLSH 2, which will provide for the payment by us to MLSH 1 and MLSH 2, collectively, of 85% of the amount of tax benefits, if any, that we actually realize, or in some circumstances are deemed to realize, as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement. Due to the uncertainty of various factors, we cannot estimate the likely tax benefits we will realize as a result of purchases of LLC Units and LLC Unit exchanges, and the resulting amounts we are likely to pay out to MLSH 1 and MLSH 2 pursuant to
62
the Tax Receivable Agreement; however, we estimate that such payments may be substantial. See “Organizational Structure—Tax Receivable Agreement.” Although we will retain 15% of the amount of such tax benefits, this and other aspects of our organizational structure may adversely impact the future trading market for the Class A common stock.
We may not be able to realize all or a portion of the tax benefits that are currently expected to result from the tax attributes covered by the Tax Receivable Agreement and from payments made under the Tax Receivable Agreement.
Our ability to realize the tax benefits that we currently expect to be available as a result of the attributes covered by the Tax Receivable Agreement, the payments made pursuant to the Tax Receivable Agreement, and the interest deductions imputed under the Tax Receivable Agreement all depend on a number of assumptions, including that we earn sufficient taxable income each year during the period over which such deductions are available and that there are no adverse changes in applicable law or regulations. Additionally, if our actual taxable income were insufficient or there were additional adverse changes in applicable law or regulations, we may be unable to realize all or a portion of the expected tax benefits and our cash flows and shareholders’ equity could be negatively affected. See “Organizational Structure—Tax Receivable Agreement.”
In certain circumstances, Topco LLC will be required to make distributions to us and MLSH 1 and the distributions may be substantial.
Topco LLC is treated as a partnership for U.S. federal income tax purposes and, as such, is not subject to U.S. federal income tax. Instead, taxable income is allocated to its members, including us. We intend to cause Topco LLC to make tax distributions quarterly to the LLC Unitholders (including us), in each case on a pro rata basis based on Topco LLC’s net taxable income and without regard to any applicable basis adjustment under Section 743(b) of the Code. Funds used by Topco LLC to satisfy its tax distribution obligations will not be available for reinvestment in our business. Moreover, these tax distributions may be substantial, and will likely exceed (as a percentage of Topco LLC’s income) the overall effective tax rate applicable to a similarly situated corporate taxpayer. As a result, it is possible that we will receive distributions significantly in excess of our tax liabilities and obligations to make payments under the Tax Receivable Agreement. While our Board may choose to distribute such cash balances as dividends on our Class A common stock, they will not be required to do so, and may in their sole discretion choose to use such excess cash for any purpose depending upon the facts and circumstances at the time of determination. See “Dividend Policy.”
Unanticipated changes in effective tax rates or adverse outcomes resulting from examination of our income or other tax returns could adversely affect our operating results and financial condition.
We are subject to income taxes in the United States, Canada and the U.K. Our tax liabilities will be subject to the allocation of expenses in differing jurisdictions. Our future effective tax rates could be subject to volatility or adversely affected by a number of factors, including:
| • | changes in the valuation of our deferred tax assets and liabilities; |
| • | expected timing and amount of the release of any tax valuation allowances; |
| • | expiration of, or detrimental changes in, research and development tax credit laws; or |
| • | changes in tax laws, regulations or interpretations thereof. |
In addition, we may be subject to audits of our income, sales and other transaction taxes by U.S. federal, state and foreign authorities. Outcomes from these audits could have an adverse effect on our operating results and financial condition.
63
If we were deemed to be an investment company under the Investment Company Act of 1940, as amended (the “1940 Act”), applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Under Sections 3(a)(1)(A) and (C) of the 1940 Act, a company generally will be deemed to be an “investment company” for purposes of the 1940 Act if it (1) is, or holds itself out as being, engaged primarily, or proposes to engage primarily, in the business of investing, reinvesting or trading in securities or (2) is engaged, or proposes to engage, in the business of investing, reinvesting, owning, holding or trading in securities and it owns or proposes to acquire investment securities having a value exceeding 40% of the value of its total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis. We do not believe that we are an “investment company,” as such term is defined in either of those sections of the 1940 Act.
As the sole managing member of Topco LLC, we will control and manage Topco LLC. On that basis, we believe that our interest in Topco LLC is not an “investment security” under the 1940 Act. Therefore, we have less than 40% of the value of our total assets (exclusive of U.S. government securities and cash items) in “investment securities.” However, if we were to lose the right to manage and control Topco LLC, interests in Topco LLC could be deemed to be “investment securities” under the 1940 Act.
We intend to conduct our operations so that we will not be deemed to be an investment company. However, if we were deemed to be an investment company, restrictions imposed by the 1940 Act, including limitations on our capital structure and our ability to transact with affiliates, could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Risks Related to Our Class A Common Stock and This Offering
GTCR controls us, and its interests may conflict with ours or yours in the future.
Immediately following this offering, investment entities affiliated with GTCR will control approximately 81% of the voting power of our outstanding common stock, or 78% if the underwriters exercise in full their option to purchase additional shares, which means that, based on its percentage voting power controlled after the offering, GTCR will control the vote of all matters submitted to a vote of our shareholders. This control will enable GTCR to control the election of the members of the Board and all other corporate decisions. Even when GTCR ceases to control a majority of the total voting power, for so long as GTCR continues to own a significant percentage of our common stock, GTCR will still be able to significantly influence the composition of our Board and the approval of actions requiring shareholder approval. Accordingly, for such period of time, GTCR will have significant influence with respect to our management, business plans and policies, including the appointment and removal of our officers, decisions on whether to raise future capital and amending our charter and bylaws, which govern the rights attached to our common stock. In particular, for so long as GTCR continues to own a significant percentage of our common stock, GTCR will be able to cause or prevent a change of control of us or a change in the composition of our Board and could preclude any unsolicited acquisition of us. The concentration of ownership could deprive you of an opportunity to receive a premium for your shares of Class A common stock as part of a sale of us and ultimately might affect the market price of our Class A common stock.
In addition, in connection with this offering, we will enter into a Director Nomination Agreement with GTCR that provides GTCR the right to nominate to the Board a number of designees equal to at least: (i) 100% of the total number of directors comprising the Board, so long as GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 40% of the total amount of shares of Class A common stock and Class B common stock it beneficially owns as of the date of this offering, (ii) 40% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 30% but less than 40% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering, (iii) 30% of the total number of directors,
64
in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 20% but less than 30% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering, (iv) 20% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 10% but less than 20% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering and (v) one director, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 5% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering. The Director Nomination Agreement will also provide that GTCR may assign such right to a GTCR affiliate. The Director Nomination Agreement will prohibit us from increasing or decreasing the size of our Board without the prior written consent of GTCR. See “Certain Relationships and Related Party Transactions—Director Nomination Agreement” for more details with respect to the Director Nomination Agreement.
GTCR and its affiliates engage in a broad spectrum of activities, including investments in our industry generally. In the ordinary course of their business activities, GTCR and its affiliates may engage in activities where their interests conflict with our interests or those of our other shareholders, such as investing in or advising businesses that directly or indirectly compete with certain portions of our business or are suppliers or customers of ours. Our certificate of incorporation to be effective at or prior to the consummation of this offering will provide that none of GTCR, any of its affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his or her director and officer capacities) or its affiliates will have any duty to refrain from engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. GTCR also may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. In addition, GTCR may have an interest in pursuing acquisitions, divestitures and other transactions that, in its judgment, could enhance its investment, even though such transactions might involve risks to you or may not prove beneficial.
Upon listing of our shares of Class A common stock on The Nasdaq Global Select Market, we will be a “controlled company” within the meaning of the rules of NASDAQ and, as a result, we will qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections as those afforded to shareholders of companies that are subject to such governance requirements.
After completion of this offering, GTCR will continue to control a majority of the voting power of our outstanding common stock. As a result, we will be a “controlled company” within the meaning of the corporate governance standards of NASDAQ. Under these rules, a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including:
| • | the requirement that a majority of our Board consist of independent directors; |
| • | the requirement that we have a nominating and corporate governance committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; |
| • | the requirement that we have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| • | the requirement for an annual performance evaluation of the nominating and corporate governance and compensation committees. |
Following this offering, we intend to utilize these exceptions. As a result, we may not have a majority of independent directors on our Board, our compensation and nominating and corporate governance committees may not consist entirely of independent directors and our compensation and nominating and corporate governance committees may not be subject to annual performance evaluations. Accordingly, you will not have
65
the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of NASDAQ.
We may allocate the net proceeds from this offering in ways that you and other shareholders may not approve.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section titled “Use of Proceeds.” Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment, and the failure by our management to apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short- and intermediate-term interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the United States government. These investments may not yield a favorable return to our shareholders. If we do not invest or apply the net proceeds from this offering in ways that enhance shareholder value, we may fail to achieve expected results, which could cause our stock price to decline.
As a result of becoming a public company, we will be obligated to develop and maintain proper and effective internal control over financial reporting in order to comply with Section 404 of the Sarbanes-Oxley Act. We may not complete our analysis of our internal control over financial reporting in a timely manner, or these internal controls may not be determined to be effective, which may adversely affect investor confidence in us and, as a result, the value of our Class A common stock.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP. We are in the very early stages of the costly and challenging process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act. We may not be able to complete our evaluation, testing and any required remediation prior to becoming a public company or in a timely manner thereafter. If we are unable to assert that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, which would cause the price of our Class A common stock to decline, and we may be subject to investigation or sanctions by the SEC.
We will be required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting as of the end of the fiscal year that coincides with the filing of our second annual report on Form 10-K. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. We will also be required to disclose changes made in our internal control and procedures on a quarterly basis. However, our independent registered public accounting firm will not be required to report on the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act until the later of the year following our first annual report required to be filed with the SEC, or the date we are no longer an “emerging growth company” as defined in the JOBS Act if we take advantage of the exemptions contained in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating.
Additionally, the existence of any material weakness or significant deficiency would require management to devote significant time and incur significant expense to remediate any such material weaknesses or significant deficiencies and management may not be able to remediate any such material weaknesses or significant deficiencies in a timely manner. The existence of any material weakness in our internal control over financial reporting could also result in errors in our financial statements that could require us to restate our financial statements, cause us to fail to meet our reporting obligations and cause shareholders to lose confidence in our
reported financial information, all of which could materially and adversely affect our business and stock price.
66
To comply with the requirements of being a public company, we may need to undertake various costly and time-consuming actions, such as implementing new internal controls and procedures and hiring accounting or internal audit staff, which may adversely affect our business, financial condition, results of operations, cash flows and prospects.
We are an “emerging growth company” and we have elected and expect to elect to comply with reduced public company reporting requirements, which could make our Class A common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we are eligible for certain exemptions from various public company reporting requirements. These exemptions include, but are not limited to, (i) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (ii) reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements, (iii) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved and (iv) not being required to provide audited financial statements for the year ended December 31, 2017 or five years of Selected Consolidated Financial Data in this prospectus. We could be an emerging growth company for up to five years after the first sale of our Class A common stock pursuant to an effective registration statement under the Securities Act, which fifth anniversary will occur in 2025. However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenue exceeds $1.07 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we would cease to be an emerging growth company prior to the end of such five-year period. We have made certain elections with regard to the reduced disclosure obligations regarding executive compensation in this prospectus and may elect to take advantage of other reduced disclosure obligations in future filings. As a result, the information that we provide to holders of our common stock may be different than you might receive from other public reporting companies in which you hold equity interests. We cannot predict if investors will find our Class A common stock less attractive as a result of reliance on these exemptions. If some investors find our Class A common stock less attractive as a result of any choice we make to reduce disclosure, there may be a less active trading market for our Class A common stock and the market price for our Class A common stock may be more volatile.
The JOBS Act also permits an emerging growth company like us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are electing to take advantage of this extended transition period for complying with new or revised accounting standards provided for by the JOBS Act. We will therefore comply with new or revised accounting standards when they apply to private companies. As a result, our financial statements may not be comparable with companies that comply with public company effective dates for accounting standards.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business, particularly after we are no longer an “emerging growth company.”
As a public company, we will incur legal, accounting and other expenses that we did not previously incur. We will become subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and the Sarbanes-Oxley Act, the listing requirements of NASDAQ and other applicable securities rules and regulations. Compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.” The Exchange Act requires that we file annual, quarterly and current reports with respect to our business, financial condition, results of operations, cash flows and prospects. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective internal controls and procedures for financial reporting. Furthermore, the need to establish the corporate infrastructure demanded of a public company may divert our management’s attention from implementing our growth strategy, which could prevent us from improving our business, financial condition,
67
results of operations, cash flows and prospects. We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a public company. However, the measures we take may not be sufficient to satisfy our obligations as a public company. In addition, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. These additional obligations could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of our management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and there could be a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Provisions of our corporate governance documents could make an acquisition of us more difficult and may prevent attempts by our shareholders to replace or remove our current management, even if beneficial to our shareholders.
Our certificate of incorporation and bylaws to be effective at or prior to the consummation of this offering and the Delaware General Corporation Law (the “DGCL”) contain provisions that could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our shareholders. Among other things:
| • | these provisions allow us to authorize the issuance of undesignated preferred stock, the terms of which may be established and the shares of which may be issued without shareholder approval, and which may include supermajority voting, special approval, dividend, or other rights or preferences superior to the rights of shareholders; |
| • | these provisions provide for a classified board of directors with staggered three-year terms; |
| • | these provisions provide that, at any time when GTCR controls, in the aggregate, less than 40% of the outstanding shares of our Class A common stock, directors may only be removed for cause, and only by the affirmative vote of holders of at least 662⁄3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class; |
| • | these provisions prohibit shareholder action by written consent from and after the date on which GTCR controls, in the aggregate, less than 35% in voting power of our stock entitled to vote generally in the election of directors; |
| • | these provisions provide that for as long as GTCR controls, in the aggregate, at least 50% in voting power of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our shareholders will require the affirmative vote of a majority in voting power of the outstanding shares of our capital stock and at any time when GTCR controls, in the aggregate, less than 50% in voting power of all outstanding shares of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by |
68
| our shareholders will require the affirmative vote of the holders of at least 662⁄3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class; and |
| • | these provisions establish advance notice requirements for nominations for elections to our Board or for proposing matters that can be acted upon by shareholders at shareholder meetings; provided, however, at any time when GTCR controls, in the aggregate, at least 10% in voting power of our stock entitled to vote generally in the election of directors, such advance notice procedure will not apply to GTCR. |
We will opt out of Section 203 of the DGCL, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any interested shareholder for a period of three years following the date on which the shareholder became an interested shareholder. However, our certificate of incorporation to be effective at or prior to the consummation of this offering will contain a provision that provides us with protections similar to Section 203, and will prevent us from engaging in a business combination with a person (excluding GTCR and any of its direct or indirect transferees and any group as to which such persons are a party) who acquires at least 85% of our common stock for a period of three years from the date such person acquired such common stock, unless board or shareholder approval is obtained prior to the acquisition. See “Description of Capital Stock—Anti-Takeover Provisions.” These provisions could discourage, delay or prevent a transaction involving a change in control of our company. These provisions could also discourage proxy contests and make it more difficult for you and other shareholders to elect directors of your choosing and cause us to take other corporate actions you desire, including actions that you may deem advantageous, or negatively affect the trading price of our Class A common stock. In addition, because our Board is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our shareholders to replace current members of our management team.
These and other provisions in our certificate of incorporation, bylaws and Delaware law could make it more difficult for shareholders or potential acquirers to obtain control of our Board or initiate actions that are opposed by our then-current Board, including actions to delay or impede a merger, tender offer or proxy contest involving our company. The existence of these provisions could negatively affect the price of our Class A common stock and limit opportunities for you to realize value in a corporate transaction.
For information regarding these and other provisions, see “Description of Capital Stock.”
Our certificate of incorporation will designate the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation that may be initiated by our shareholders and the federal district courts of the United States as the exclusive forum for litigation arising under the Securities Act, which could limit our shareholders’ ability to obtain a favorable judicial forum for disputes with us.
Pursuant to our certificate of incorporation, which we will adopt at or prior to the consummation of this offering, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for any claims in state court for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our shareholders, (3) any action asserting a claim against us arising pursuant to any provision of the DGCL, our certificate of incorporation or our bylaws or (4) any other action asserting a claim against us that is governed by the internal affairs doctrine; provided that for the avoidance of doubt, the forum selection provision that identifies the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation, including any “derivative action,” will not apply to suits to enforce a duty or liability created by the Securities Act, the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our certificate of incorporation will also provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Our certificate of
69
incorporation will further provide that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock is deemed to have notice of and consented to the provisions of our certificate of incorporation described above. See “Description of Capital Stock—Forum Selection.” The forum selection provisions in our certificate of incorporation may have the effect of discouraging lawsuits against us or our directors and officers and may limit our shareholders’ ability to obtain a favorable judicial forum for disputes with us. If the enforceability of our forum selection provisions were to be challenged, we may incur additional costs associated with resolving such challenge. While we currently have no basis to expect any such challenge would be successful, if a court were to find our forum selection provisions to be inapplicable or unenforceable with respect to one or more of these specified types of actions or proceedings, we may incur additional costs associated with having to litigate in other jurisdictions, which could have an adverse effect on our business, financial condition, results of operations, cash flows and prospects and result in a diversion of the time and resources of our employees, management and board of directors.
If you purchase shares of Class A common stock in this offering, you will suffer immediate and substantial dilution of your investment.
The initial public offering price of our Class A common stock is substantially higher than the net tangible book value per share of our Class A common stock. Therefore, if you purchase shares of our Class A common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. Based on an assumed initial public offering price of $25.50 per share, the mid-point of the price range set forth on the cover page of this prospectus, you will experience immediate dilution of $26.73 per share, representing the difference between our pro forma net tangible book value per share at September 30, 2020 after giving effect to this offering and the initial public offering price. In addition, purchasers of Class A common stock in this offering will have contributed 88% of the aggregate price paid by all purchasers of our Class A common stock but will own only approximately 61% of our Class A common stock outstanding after this offering. See “Dilution” for more detail.
An active, liquid trading market for our Class A common stock may not develop, which may limit your ability to sell your shares.
Prior to this offering, there was no public market for our Class A common stock. Although our Class A common stock has been approved for listing on The Nasdaq Global Select Market under the trading symbol “MRVI,” an active trading market for our Class A common stock may never develop or be sustained following this offering. The initial public offering price will be determined by negotiations between us and the underwriters and may not be indicative of market prices of our Class A common stock that will prevail in the open market after the offering. A public trading market having the desirable characteristics of depth, liquidity and orderliness depends upon the existence of willing buyers and sellers at any given time, such existence being dependent upon the individual decisions of buyers and sellers over which neither we nor any market maker has control. The failure of an active and liquid trading market to develop and continue would likely have a material adverse effect on the value of our Class A common stock. The market price of our Class A common stock may decline below the initial public offering price, and you may not be able to sell your shares of our Class A common stock at or above the price you paid in this offering, or at all. An inactive market may also impair our ability to raise capital to continue to fund operations by issuing additional shares of our Class A common stock or other equity or equity-linked securities and may impair our ability to acquire other companies or technologies by using any such securities as consideration.
Our operating results and stock price may be volatile, and the market price of our Class A common stock after this offering may drop below the price you pay.
Our quarterly operating results are likely to fluctuate in the future. In addition, securities markets worldwide have experienced, and are likely to continue to experience, significant price and volume fluctuations, including as a result of the COVID-19 pandemic. This market volatility, as well as general economic, market or political
70
conditions, could subject the market price of our Class A common stock to wide price fluctuations regardless of our operating performance. Our operating results and the trading price of our Class A common stock may fluctuate in response to various factors, including:
| • | market conditions in our industry or the broader stock market; |
| • | actual or anticipated fluctuations in our quarterly financial and operating results; |
| • | introduction of new products or services by us or our competitors; |
| • | issuance of new or changed securities analysts’ reports or recommendations; |
| • | sales, or anticipated sales, of large blocks of our stock; |
| • | additions or departures of key personnel; |
| • | regulatory or political developments; |
| • | litigation and governmental investigations; |
| • | changing economic conditions; |
| • | investors’ perception of us; |
| • | events beyond our control such as weather, war and health crises such as the COVID-19 pandemic; and |
| • | any default on our indebtedness. |
These and other factors, many of which are beyond our control, may cause our operating results and the market price and demand for our Class A common stock to fluctuate substantially. Fluctuations in our quarterly operating results could limit or prevent investors from readily selling their shares of Class A common stock and may otherwise negatively affect the market price and liquidity of our shares of Class A common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the company that issued the stock. If any of our shareholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management from our business, which could significantly harm our profitability and reputation.
A significant portion of our total outstanding shares of Class A common stock are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our Class A common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our Class A common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares of Class A common stock intend to sell shares, could reduce the market price of our Class A common stock. After this offering, we will have 81,379,818 outstanding shares of Class A common stock based on the number of shares outstanding as of September 30, 2020. This includes shares of Class A common stock that we are selling in this offering, which may be resold in the public market immediately unless purchased by one of our affiliates. Following the consummation of this offering, substantially all of the shares that are not being sold in this offering will be subject to a 180-day lock-up period provided under lock-up agreements executed in connection with this offering described in “Underwriters” and restricted from immediate resale under the federal securities laws as described in “Shares Eligible for Future Sale.” All of these shares of Class A common stock will, however, be able to be resold after the expiration of the lock-up period, as well as pursuant to customary exceptions thereto or upon the waiver of the lock-up agreement by the representatives on behalf of the underwriters. We also intend to register shares of Class A common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to the lock-up agreements. As restrictions on resale end, the market price of our stock could decline if the holders of currently restricted shares of Class A common stock sell them or are perceived by the market as intending to sell them.
71
Because we have no current plans to pay regular cash dividends on our Class A common stock following this offering, you may not receive any return on investment unless you sell your Class A common stock for a price greater than that which you paid for it.
We do not anticipate paying any regular cash dividends on our Class A common stock following this offering. Any decision to declare and pay dividends in the future will be made at the discretion of our Board and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that our Board may deem relevant. In addition, our ability to pay dividends is, and may be, limited by covenants of existing and any future outstanding indebtedness we or our subsidiaries incur, including under our New Credit Agreement. Therefore, any return on investment in our Class A common stock is solely dependent upon the appreciation of the price of our Class A common stock on the open market, which may not occur. See “Dividend Policy” for more detail.
If securities or industry analysts do not publish research or reports about our business, if they publish unfavorable research or reports, or adversely change their recommendations regarding our Class A common stock or if our results of operations do not meet their expectations, our stock price and trading volume could decline.
If a trading market for our Class A common stock develops, the trading market will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not have any control over these analysts. As a newly public company, we may be slow to attract research coverage. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us provide inaccurate or unfavorable research, issue an adverse opinion regarding our stock price or if our results of operations do not meet their expectations, our stock price could decline. Moreover, if one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
We may issue shares of preferred stock in the future, which could make it difficult for another company to acquire us or could otherwise adversely affect holders of our Class A common stock, which could depress the price of our Class A common stock.
Our certificate of incorporation will authorize us to issue one or more series of preferred stock. Our Board will have the authority to determine the preferences, limitations and relative rights of the shares of preferred stock and to fix the number of shares constituting any series and the designation of such series, without any further vote or action by our shareholders. Our preferred stock could be issued with voting, liquidation, dividend and other rights superior to the rights of our Class A common stock. The potential issuance of preferred stock may delay or prevent a change in control of us, discouraging bids for our Class A common stock at a premium to the market price, and materially adversely affect the market price and the voting and other rights of the holders of our Class A common stock.
72
This prospectus contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties. All statements other than statements of historical fact included in this prospectus are forward-looking statements. Forward-looking statements give our current expectations and projections relating to our financial condition, results of operations, plans, objectives, future performance and business. You can identify forward-looking statements by the fact that they do not relate strictly to historical or current facts. These statements may include words such as “anticipate,” “estimate,” “expect,” “project,” “plan,” “intend,” “believe,” “may,” “will,” “should,” “can have,” “likely” and other words and terms of similar meaning in connection with any discussion of the timing or nature of future operating or financial performance or other events. For example, all statements we make relating to our estimated and projected costs, expenditures, cash flows, growth rates and financial results, our plans and objectives for future operations, growth or initiatives, strategies or the expected outcome or impact of pending or threatened litigation are forward-looking statements. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected, including:
| • | our history of losses, the risk that we may continue to incur losses in the future and our ability to generate sufficient revenue to achieve or maintain profitability; |
| • | the fluctuation of our operating results, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide; |
| • | our dependence on a limited number of customers for a high percentage of our revenue; |
| • | the use of certain of our products in the production of vaccines and therapies that represent relatively new and still-developing modes of treatment, which may experience unforeseen adverse events, negative clinical outcomes or increased regulatory scrutiny; |
| • | the impact of COVID-19 and any pandemic, epidemic or outbreak of infectious disease; |
| • | changes in economic conditions; |
| • | our dependence on customers’ spending on and demand for outsourced nucleic acid production, biologics safety testing and protein detection research products and services; |
| • | competition with life science, pharmaceutical and biotechnology companies who are substantially larger than we are and potentially capable of developing new approaches that could make our products, services and technologies obsolete; |
| • | the ability of our products and services to perform as expected and the reliability of the technology on which our products and services are based; |
| • | the complexity of our products and the fact that they are subject to quality control requirements; |
| • | our reliance on a limited number of suppliers or, in some cases, sole suppliers, for some of our raw materials and our inability to find replacements or immediately transition to alternative suppliers; |
| • | our dependence on a stable and adequate supply of quality raw materials from our suppliers, and the risk of adverse impacts from price increases or interruptions of such supply; |
| • | disruptions at our sites; |
| • | our ability to manufacture in specific quantities; |
| • | natural disasters, geopolitical unrest, war, terrorism, public health issues such as COVID-19 or other catastrophic events that could disrupt the supply, delivery or demand of products and services; |
| • | our ability to secure additional financing for future strategic transactions; |
| • | our reliance on third-party package delivery services and adverse impacts arising from significant disruptions of these services, damages or losses sustained during shipping or significant increases in prices; |
73
| • | our ability to continue to hire and retain skilled personnel; |
| • | our ability to successfully identify and implement distribution arrangements and marketing alliances; |
| • | the market acceptance of our life science reagents; |
| • | the market receptivity to our new products and services upon their introduction; |
| • | our ability to implement our strategies for revenue growth; |
| • | the accuracy of our estimates of market opportunity and forecasts of market growth included in this prospectus; |
| • | product liability lawsuits; |
| • | the application of privacy laws, security laws, regulations, policies and contractual obligations related to data privacy and security; |
| • | our ability to efficiently manage our growth; |
| • | the success of any opportunistic acquisitions; |
| • | the integrity of our internal computer systems; |
| • | the impact of export and import control laws and regulations; |
| • | risks related to Brexit; |
| • | changes in political, economic or governmental regulations; |
| • | financial, operating, legal and compliance risks associated with global operations; |
| • | risks associated with our acquisitions; |
| • | impacts from foreign currency exchange rates; |
| • | the risk that our products could become subject to more onerous regulation in the future; |
| • | our ability to use net operating loss and tax credit carryforwards; |
| • | the fact that our activities are and will continue to be subject to extensive government regulation; |
| • | the risk that we may be required to record a significant charge to earnings if our goodwill or other amortizable intangible assets become impaired; |
| • | unfavorable accounting charges or effects driven by changes in accounting principles or guidance; |
| • | impacts on our financial results from our revenue recognition and other factors; |
| • | fluctuations in our effective tax rate; |
| • | environmental risks; |
| • | our ability to obtain, maintain and enforce intellectual property protection for our current and future products; |
| • | our ability to protect the confidentiality of our proprietary information; |
| • | risks associated with lawsuits to protect our patents or with respect to the infringement, misappropriations or other violations of intellectual property rights of third parties; |
| • | risks associated with failures to comply with our obligations under license agreements; |
| • | potential changes in patent law in the United States and other jurisdictions; |
| • | our ability to obtain and maintain our patent protection; |
74
| • | impact of claims by third parties that we or our employees, consultants or independent contractors have infringed, misappropriated or otherwise violated their intellectual property; |
| • | our ability to protect our intellectual property and proprietary rights throughout the world; |
| • | our reliance on confidentiality agreements; |
| • | our ability to protect our trademarks and trade names; |
| • | threats not related to intellectual property; and |
| • | other risks addressed under the heading “Risk Factors” herein. |
We derive many of our forward-looking statements from our operating budgets and forecasts, which are based on many detailed assumptions. While we believe that our assumptions are reasonable, we caution that it is very difficult to predict the impact of known factors, and it is impossible for us to anticipate all factors that could affect our actual results. Important factors that could cause actual results to differ materially from our expectations, or cautionary statements, are disclosed under the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this prospectus. All written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by these cautionary statements as well as other cautionary statements that are made from time to time in our other SEC filings and public communications. You should evaluate all forward-looking statements made in this prospectus in the context of these risks and uncertainties.
We caution you that the important factors referenced above may not contain all of the factors that are important to you. In addition, we cannot assure you that we will realize the results or developments we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our operations in the way we expect. The forward-looking statements included in this prospectus are made only as of the date hereof. We undertake no obligation to update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law.
75
Unless otherwise indicated, information in this prospectus concerning economic conditions, our industry, our markets and our competitive position is based on a variety of sources, including information from independent industry analysts and publications, as well as our own estimates and research. This information involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While we are responsible for all disclosure in this prospectus, and we believe the information presented in this prospectus is generally reliable, forecasts, assumptions, expectations, beliefs, estimates and projects involve risk and uncertainties and are subject to change based on various factors, including those described under “Forward-Looking Statements” and “Risk Factors.”
76
We estimate, based upon an assumed initial public offering price of $25.50 per share (which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), we will receive net proceeds from this offering of approximately $1,184.5 million (or $1,365.2 million if the underwriters exercise their option to purchase additional shares in full, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use such net proceeds as follows:
| • | $94.5 million to acquire 3,921,569 newly-issued LLC Units in Topco LLC and $942.7 million to acquire 39,121,430 outstanding LLC Units (or $1,096.2 million to acquire 45,489,067 LLC Units if the underwriters exercise their option to purchase additional shares in full) from MLSH 1, in each case at a purchase price per LLC Unit equal to the initial public offering price per share of Class A common stock in this offering, less underwriting discounts and commissions; and |
| • | $167.6 million to pay MLSH 2 as consideration for the Blocker Mergers and, if the underwriters exercise their option to purchase additional shares in full, $27.3 million to acquire outstanding shares of Class A common stock from MLSH 2 at a purchase price per share equal to the initial public offering price per share of Class A common stock in this offering, less underwriting discounts and commissions. |
In turn, Topco LLC intends to apply the balance of the proceeds it receives from us (including any additional proceeds it may receive from us if the underwriters exercise their option to purchase additional shares of Class A common stock) to (i) repay $50.0 million of our outstanding indebtedness under the New Credit Agreement, under which $600.0 million was outstanding and which had an interest rate of 5.25% as of October 31, 2020, (ii) pay expenses incurred in connection with this offering and the Organizational Transactions and (iii) for general corporate purposes.
Pending use of the net proceeds from this offering described above, we may invest the net proceeds in short- and intermediate-term interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the United States government.
Assuming no exercise of the underwriters’ option to purchase additional shares, each $1.00 increase or decrease in the assumed initial public offering price of $25.50 per share (which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) would increase or decrease the net proceeds to us from this offering by approximately $47.3 million, assuming the number of shares of Class A common stock offered, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Each 1,000,000 increase or decrease in the number of shares of Class A common stock offered in this offering would increase or decrease the net proceeds to us from this offering by approximately $24.1 million, assuming that the initial public offering price per share for the offering remains at $25.50 (which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
77
We currently intend to retain all available funds and any future earnings to fund the development and growth of our business and to repay indebtedness and, therefore, we do not anticipate paying any cash dividends in the foreseeable future. Additionally, because we are a holding company, our ability to pay dividends on our Class A common stock may be limited by restrictions on the ability of our subsidiaries to pay dividends or make distributions to us. Any future determination to pay dividends will be at the discretion of our Board, subject to compliance with covenants in current and future agreements governing our and our subsidiaries’ indebtedness, including our New Credit Agreement, and will depend on our results of operations, financial condition, capital requirements and other factors that our Board deems relevant.
Under the terms of the LLC Operating Agreement, Topco LLC is obligated to make tax distributions to current and future unitholders, including us and shall be made on a pro rata basis among the LLC Unitholders based on Topco LLC’s net taxable income and without regard to any applicable basis adjustment under Section 743(b) of the Code. These tax distributions may be substantial, and will likely exceed (as a percentage of Topco LLC’s income) the overall effective tax rate applicable to a similarly situated corporate taxpayer. As a result, it is possible that we will receive distributions significantly in excess of our tax liabilities and obligations to make payments under the Tax Receivable Agreement. While our Board may choose to distribute such cash balances as dividends on our Class A common stock (subject to the limitations set forth in the preceding paragraph), they will not be required to do so, and may in its sole discretion choose to use such excess cash for any purpose depending upon the facts and circumstances at the time of determination.
78
The following table describes our cash and consolidated capitalization as of September 30, 2020:
| • | of Topco LLC and its subsidiaries on an actual historical basis; |
| • | of Maravai LifeSciences Holdings, Inc. on a pro forma basis, after giving effect to the Organizational Transactions; and |
| • | of Maravai LifeSciences Holdings, Inc. on a pro forma as adjusted basis, after giving effect to the Organizational Transactions, our sale of 50,000,000 shares of Class A common stock in this offering at an assumed initial public offering price of $25.50 per share (which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us (assuming no exercise of the underwriters’ option to purchase additional shares) and the application of the net proceeds of the offering as set forth in “Use of Proceeds.” |
You should read this table in conjunction with the unaudited condensed consolidated financial statements and the related notes, “Use of Proceeds,” “Organizational Structure,” “Unaudited Pro Forma Condensed Consolidated Financial Information” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| As of September 30, 2020 | ||||||||||||
| (unaudited) | ||||||||||||
| (dollars in thousands, except share data and par value) | Historical Topco LLC |
Pro Forma for the Organizational Transactions |
Pro Forma As Adjusted for the Organizational Transactions and the Offering |
|||||||||
| Cash |
$ | 124,882 | $ | 127,157 | $ | 171,657 | ||||||
|
|
|
|
|
|
|
|||||||
| Indebtedness: |
||||||||||||
| Credit Facilities(1) |
$ | 351,704 | $ | 584,600 | $ | 534,600 | ||||||
|
|
|
|
|
|
|
|||||||
| Class A common stock, $0.01 par value per share, no shares authorized, issued or outstanding, on an actual basis; 500,000,000 shares authorized, 31,379,818 shares issued and outstanding, on a pro forma basis; 500,000,000 shares authorized; 81,379,818 shares issued and outstanding, on a pro forma as adjusted basis |
— | 314 | 814 | |||||||||
| Class B common stock, $0.01 par value per share, no shares authorized, issued or outstanding, on an actual basis; 300,000,000 shares authorized; 176,458,692 shares issued and outstanding, on a pro forma basis; 300,000,000 shares authorized; 176,458,692 shares issued and outstanding, on a pro forma as adjusted basis |
— | 1,765 | 1,765 | |||||||||
| Additional paid in capital |
— | 58,550 | 105,298 | |||||||||
| Contributed capital |
14,777 | — | — | |||||||||
| Accumulated other comprehensive loss |
(164 | ) | (25 | ) | (52 | ) | ||||||
| Retained earnings (deficit) |
21,381 | (60,706 | ) | (61,373 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total member’s/shareholders’ equity (deficit) |
35,994 | (102 | ) | 46,452 | ||||||||
| Non-controlling interests(2) |
— | (12,267 | ) | 43,239 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 387,698 | $ | 572,231 | $ | 624,291 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Includes (a) a first lien credit agreement (the “First Lien Credit Agreement”) entered into on August 2, 2018 and providing for a $250.0 million first lien term loan facility (the “First Lien Term Loan”) and a $50.0 million revolving credit facility (the “Revolving Credit Facility”) and (b) a second lien credit agreement (the “Second Lien Credit Agreement”) providing for a $100.0 million second lien term loan facility (the “Second Lien Term Loan” and, together with the First Lien Term Loan and the Revolving |
79
| Credit Facility, the “Credit Facilities”), and reflects $8.3 million of unamortized debt issuance costs. On October 19, 2020, we entered into the New Credit Agreement, which provides for a $600.0 million term loan and a $180.0 million revolving credit facility. The proceeds from the borrowings under the New Credit Agreement were used in part to repay $363.0 million of pre-existing debt and accrued interest under the Credit Facilities, make a distribution of $88.6 million to MLSH 1 and repurchase incentive units and noncontrolling interests of $9.1 million and $120.0 million, respectively, in one of our subsidiaries. On a pro forma as adjusted basis, the amount reflects the repayment of $50.0 million of the amount outstanding under the New Credit Agreement with a portion of the net proceeds of the offering. |
| (2) | On a pro forma as adjusted basis, includes the Topco LLC interests not owned by us, which represents 68% of Topco LLC’s LLC Units. MLSH 1 will hold the non-controlling economic interest in Topco LLC. Maravai LifeSciences Holdings, Inc. will hold 32% of the economic interest in Topco LLC. |
A $1.00 increase or decrease in the assumed initial public offering price of $25.50 per share (which is the midpoint of the price range set forth on the cover page of this prospectus) would increase or decrease each of cash, total shareholders’ equity and total capitalization on a pro forma basis by approximately $0.0 million, assuming the number of shares of Class A common stock offered, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each 1,000,000 increase or decrease in the number of shares of Class A common stock offered in this offering would increase or decrease each of cash, total shareholders’ equity and total capitalization on a pro forma basis by approximately $0.0 million, based on an assumed initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The number of shares of Class A common stock to be outstanding after the completion of this offering, excludes 176,458,692 shares of Class A common stock that may be issuable upon exercise of redemption and exchange rights held by MLSH 1, 10,313,540 shares of Class A common stock reserved for future issuance under our ESPP and 25,783,851 shares of Class A common stock reserved for future issuance under the 2020 Plan, including (i) options to purchase 1,584,400 shares of Class A common stock that we will issue to certain employees upon completion of this offering, with an exercise price set at the initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover of this prospectus, and that vest in accordance with the schedule described in “Executive Compensation—Actions Taken in 2020 or in Connection with This Offering—Stock Option Grants” and (ii) 75,294 RSUs that may be settled for an equal number of shares of Class A common stock that we will issue to our six independent directors upon completion of this offering and that vest annually over three years.
80
Because MLSH 1 does not own any Class A common stock or other economic interests in Maravai LifeSciences Holdings, Inc., we have presented dilution in pro forma net tangible book value per share after this offering assuming that MLSH 1 had all of its LLC Units redeemed or exchanged for newly-issued shares of Class A common stock on a one-for-one basis (rather than for cash and based upon an assumed offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) and the cancellation for no consideration of all of its shares of Class B common stock (which are not entitled to receive distributions or dividends, whether cash or stock, from Maravai LifeSciences Holdings, Inc.) in order to more meaningfully present the dilutive impact to the investors in this offering. We refer to the assumed redemption or exchange of all LLC Units for shares of Class A common stock as described in the previous sentence as the “Assumed Redemption.”
Dilution results from the fact that the initial public offering price per share of the Class A common stock is substantially in excess of the pro forma net tangible book value per share of Class A common stock after this offering. Net tangible book value (deficit) per share represents the amount of our total tangible assets less total liabilities, divided by the number of shares of Class A common stock outstanding. If you invest in our Class A common stock, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share of our Class A common stock and the pro forma net tangible book value per share of our Class A common stock after this offering.
Pro forma net tangible book value per share is determined at any date by subtracting our total liabilities from the total book value of our tangible assets and dividing the difference by the number of shares of Class A common stock, after giving effect to the Organizational Transactions, including the sale of 50,000,000 shares of Class A common stock in this offering at the assumed initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, and the Assumed Redemption. Our pro forma net tangible book value (deficit) after this offering as of September 30, 2020 was $(317.4) million, or $(1.23) per share of Class A common stock. This represents an immediate increase in our net tangible book value to MLSH 1 and MLSH 2 of $0.79 per share and an immediate dilution to new investors in this offering of $26.73 per share. We determine dilution by subtracting the pro forma net tangible book value per share after this offering from the amount of cash that a new investor paid for a share of Class A common stock. The following table illustrates this dilution:
| Assumed initial public offering price per share |
$ | 25.50 | ||||||
| Pro forma net tangible book value (deficit) per share as of September 30, 2020 prior to this offering(1) |
$ | (2.02 | ) | |||||
| Increase in net tangible book value per share attributable to the investors in this offering |
$ | 0.79 | ||||||
|
|
|
|||||||
| Pro forma net tangible book value (deficit) per share after this offering |
$ | (1.23 | ) | |||||
|
|
|
|||||||
| Dilution in net tangible book value per share to the investors in this offering |
$ | 26.73 | ||||||
|
|
|
| (1) | The computation of pro forma net tangible book value per share as of September 30, 2020 before this offering is set forth below: |
| (in thousands, except per share data) |
||||
| Book value of tangible assets(a) |
$ | 339,467 | ||
| Less: total liabilities(a) |
$ | 759,246 | ||
| Pro forma net tangible book value(a) |
$ | (419,779 | ) | |
|
|
|
|||
| Shares of Class A common stock outstanding(a) |
207,839 | |||
|
|
|
|||
| Pro forma net tangible book value per share prior to this offering |
$ | (2.02 | ) | |
|
|
|
|||
| (a) | Gives pro forma effect to the debt refinancing and partial MLSC minority interest buyout, as well as, the Organizational Transactions (other than this offering) and the Assumed Redemption. |
81
A $1.00 increase in the assumed initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, would increase pro forma net tangible book value by $11.6 million, or $0.00 per share, and would increase the dilution per share to the investors in this offering by $1.00 based on the assumptions set forth above. A $1.00 decrease in the assumed initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, would decrease pro forma net tangible book value by $13.6 million, or $0.00 per share, and would decrease the dilution per share to the investors in this offering by $1.00 based on the assumptions set forth above.
The following table summarizes as of September 30, 2020, after giving effect to the Organizational Transactions (including this offering), the number of shares of Class A common stock purchased from us, the total consideration paid and the average price per share paid by MLSH 1 and MLSH 2 and by the purchasers in this offering, based upon an assumed initial public offering price of $25.50 per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) and before deducting estimated underwriting discounts and commissions and offering expenses, after giving effect to the Assumed Redemption:
| Shares of Class A Common Stock Purchased |
Total Consideration | |||||||||||||||||||
| Number | Percent | Amount | Percent | Average Price Per Share |
||||||||||||||||
| Existing owners(1) |
207,838,510 | 80.6 | % | $ | 45,698,000 | 3.5 | % | $ | 0.22 | |||||||||||
| Investors in this offering |
50,000,000 | 19.4 | 1,275,000,000 | 96.5 | 25.50 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
257,838,510 | 100 | % | $ | 1,320,698,000 | 100 | % | $ | 5.12 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | The total consideration provided by the existing owners does not give effect to the $167,646,000 liability payable to MLSH 2 resulting from the Blocker Mergers consummated immediately prior to the completion of this offering. This liability will be satisfied with Class A common shares equal to the fair value of the Blocker Mergers less the amount of proceeds from this offering paid as consideration. |
The discussion and tables above assume no exercise of the underwriters’ option to purchase additional shares. In addition, the discussion and tables above exclude shares of Class B common stock, because holders of the Class B common stock are not entitled to distributions or dividends, whether cash or stock, from Maravai LifeSciences Holdings, Inc. If the underwriters’ option to purchase additional shares is exercised in full, after giving effect to the Assumed Redemption, MLSH 1 and MLSH 2 would own approximately 77.7% and the investors in this offering would own approximately 22.3% of the total number of shares of our Class A common stock outstanding after this offering. If the underwriters exercise their option to purchase additional shares in full, after giving effect to the Assumed Redemption, the pro forma net tangible book value (deficit) per share after this offering would be $(1.23) per share, and the dilution in the pro forma net tangible book value (deficit) per share to the investors in this offering would be $26.73 per share.
The tables and calculations above are based on the number of shares of Class A common stock outstanding as of September 30, 2020 (after giving effect to the Organizational Transactions), and exclude an aggregate of 10,313,540 shares of Class A common stock reserved for issuance under our ESPP and 25,783,851 shares of Class A common stock reserved for issuance under our 2020 Plan, each of which we expect to adopt in connection with this offering, including (i) options to purchase 1,584,400 shares of Class A common stock that we will issue to certain employees upon completion of this offering, with an exercise price set at the initial public offering price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover of this prospectus, and that vest in accordance with the schedule described in “Executive Compensation—Actions Taken in 2020 or in Connection with This Offering—Stock Option Grants” and (ii) 75,294 RSUs that may be settled for an equal number of shares of Class A common stock that we will issue to our six independent directors upon
82
completion of this offering and that vest annually over three years. To the extent that any new options or other equity incentive grants are issued in the future with an exercise price or purchase price below the initial public offering price, new investors will experience further dilution.
We may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent additional capital is raised through the sale of equity or equity-linked securities, the issuance of these securities could result in further dilution to our shareholders.
83
SELECTED CONSOLIDATED FINANCIAL DATA
The following tables present, as of the dates and for the periods indicated, the selected consolidated financial data for Topco LLC and its subsidiaries. Topco LLC is the predecessor of Maravai LifeSciences Holdings, Inc. for financial reporting purposes. The selected consolidated statement of operations data for each of the years ended December 31, 2018 and 2019 and the selected consolidated balance sheet data as of December 31, 2018 and 2019 presented below have been derived from the audited consolidated financial statements and notes of Topco LLC and its subsidiaries, included elsewhere in this prospectus. The selected condensed consolidated statement of operations data for each of the nine months ended September 30, 2019 and 2020 and the selected condensed consolidated balance sheet data as of September 30, 2020 presented below have been derived from the unaudited condensed consolidated financial statements and notes of Topco LLC and its subsidiaries, included elsewhere in this prospectus. In the opinion of management, such unaudited condensed consolidated financial statements and notes include all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of such financial data. The results of operations for the periods presented below are not necessarily indicative of the results to be expected for any future period and the results for any interim period are not necessarily indicative of the results that may be expected for a full fiscal year.
The information set forth below should be read together with the “Prospectus Summary—Summary Historical and Pro Forma Consolidated Financial Data,” “Use of Proceeds,” “Capitalization,” “Unaudited Pro Forma Condensed Consolidated Financial Data,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
84
The selected consolidated financial data of Maravai LifeSciences Holdings, Inc. have not been presented as Maravai LifeSciences Holdings, Inc. is a newly incorporated entity, has had no business transactions or activities to date and had no material assets or liabilities during the periods presented in this section.
| Historical Topco LLC | Pro Forma Maravai LifeSciences Holdings, Inc. |
|||||||||||||||||||||||
| Year Ended December 31, | Nine Months Ended September 30, |
Year Ended December 31, 2019 |
Nine Months Ended September 30, 2020 |
|||||||||||||||||||||
| Consolidated Statement of Operations Data: |
2018 | 2019 | 2019 | 2020 | ||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||
| (in thousands, except per unit and per share data) | ||||||||||||||||||||||||
| Revenue |
$ | 123,833 | $ | 143,140 | $ | 107,180 | $ | 185,745 | $ | 143,140 | $ | 185,745 | ||||||||||||
| Operating Expenses: |
||||||||||||||||||||||||
| Cost of revenue |
60,765 | 66,849 | 49,019 | 56,254 | 66,849 | 56,254 | ||||||||||||||||||
| Research and development |
4,499 | 3,627 | 2,648 | 7,212 | 3,627 | 7,212 | ||||||||||||||||||
| Selling, general and administrative |
41,194 | 48,354 | 32,570 | 52,624 | 57,586 | 58,329 | ||||||||||||||||||
| Change in estimated fair value of contingent consideration |
939 | 322 | 241 | — | 322 | — | ||||||||||||||||||
| Gain on sale and leaseback transaction |
— | (19,002 | ) | — | (19,002 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
107,397 | 119,152 | 84,478 | 97,088 | 128,384 | 102,793 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income from operations |
16,436 | 23,988 | 22,702 | 88,657 | 14,756 | 82,952 | ||||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||
| Interest expense |
(27,399 | ) | (29,959 | ) | (22,240 | ) | (21,934 | ) | (29,165 | ) | (22,980 | ) | ||||||||||||
| Loss on extinguishment of debt |
(5,622 | ) | — | — | — | |||||||||||||||||||
| Other income |
87 | 118 | 95 | 132 | 118 | 132 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss before income taxes |
(16,498 | ) | (5,853 | ) | 557 | 66,855 | (14,291 | ) | 60,104 | |||||||||||||||
| Income tax expense (benefit) |
417 | (652 | ) | 308 | 2,511 | (1,559 | ) | 6,015 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (loss) income |
$ | (16,915 | ) | $ | (5,201 | ) | $ | 249 | $ | 64,344 | $ | (12,732 | ) | 54,089 | ||||||||||
| Net (loss) income attributable to noncontrolling interests |
(12,443 | ) | (731 | ) | (672 | ) | 582 | (9,335 | ) | 39,415 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (loss) income attributable to Topco LLC member |
$ | (4,472 | ) | $ | (4,470 | ) | $ | 921 | $ | 63,762 | $ | (3,397 | ) | 14,674 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (loss) income per common unit attributable to Topco LLC member—basic and diluted |
$ | (0.07 | ) | $ | (0.03 | ) | $ | (0.01 | ) | $ | 0.21 | |||||||||||||
| Weighted-average common units outstanding |
253,916,941 | 253,916,941 | 253,916,941 | 253,916,941 | ||||||||||||||||||||
| Per Share Data(1): |
||||||||||||||||||||||||
| Pro forma weighted average shares of Class A common stock outstanding: |
||||||||||||||||||||||||
| Basic |
|
84,465,072 | 84,465,072 | |||||||||||||||||||||
| Diluted |
|
84,465,072 | 84,499,582 | |||||||||||||||||||||
| Pro forma net loss available to Class A common stock per share: |
||||||||||||||||||||||||
| Basic |
|
$ | (0.04 | ) | $ | 0.18 | ||||||||||||||||||
| Diluted |
|
$ | (0.04 | ) | $ | 0.18 | ||||||||||||||||||
| Selected Other Data: |
||||||||||||||||||||||||
| Adjusted EBITDA(2) |
$ | 53,000 | $ | 62,014 | $ | 48,150 | $ | 104,840 | ||||||||||||||||
| Adjusted Free Cash Flow(3) |
$ | 49,263 | $ | 42,101 | $ | 34,713 | $ | 83,118 | ||||||||||||||||
85
| Historical Topco LLC | ||||||||||||
| As of December 31, | As of September 30, | |||||||||||
| 2018 | 2019 | 2020 | ||||||||||
| Consolidated Balance Sheet Data (at period end): | (Unaudited) | |||||||||||
| Cash |
$ | 21,866 | $ | 24,700 | $ | 124,882 | ||||||
| Working capital (deficit)(4) |
17,883 | 30,990 | (46,330 | ) | ||||||||
| Total assets |
539,676 | 577,796 | 738,112 | |||||||||
| Long-term debt, less current portion |
335,550 | 334,783 | 349,204 | |||||||||
| Total liabilities |
391,660 | 433,169 | 702,118 | |||||||||
| Total member’s equity |
148,016 | 144,627 | 35,994 | |||||||||
| (1) | See the unaudited pro forma consolidated statement of operations in “Unaudited Pro Forma Consolidated Financial Information” for the description of the assumptions underlying the pro forma net loss per share calculations. |
| (2) | Adjusted EBITDA is a supplemental measure of operating performance that is not prepared in accordance with GAAP and that does not represent, and should not be considered as, an alternative to net loss, as determined in accordance with GAAP. For a reconciliation of Adjusted EBITDA to net loss, the most directly comparable GAAP measure, see “Prospectus Summary—Summary Historical Financial and Other Data.” |
| (3) | Adjusted Free Cash Flow is a supplemental measure of operating performance that is not prepared in accordance with GAAP and that does not represent, and should not be considered as, an alternative to net loss, as determined in accordance with GAAP. For a reconciliation of Adjusted Free Cash Flow, see “Prospectus Summary—Summary Historical Financial and Other Data.” |
| (4) | We define working capital (deficit) as current assets less current liabilities. |
86
UNAUDITED CONSOLIDATED PRO FORMA FINANCIAL INFORMATION
The unaudited pro forma consolidated balance sheet as of September 30, 2020 and the unaudited pro forma consolidated statements of income for the nine months ended September 30, 2020 and the year ended December 31, 2019 present our financial position and results of operations after giving effect to the following pro forma transactions (the “Pro Forma Transactions”):
| (1) | The effects of the Company’s refinancing of its debt and the use of a portion of the proceeds to make a distribution to MLSH 1, as described under “Description of Certain Indebtedness;” |
| (2) | The effects of using a portion of the proceeds from the Company’s refinancing of its debt to repurchase minority interests at one of its subsidiaries, MLSC Holdings, LLC. |
| (3) | The Organizational Transactions described under “Organizational Structure.” |
| (4) | The effects of the tax receivable agreement, as described under “Certain Relationships and Related Party Transactions—Tax Receivable Agreement;” |
| (5) | A provision for corporate income taxes on the income attributable to the Company at a tax rate of 24.19% and 24.01% as of December 31, 2019 and September 30, 2020, inclusive of all U.S. federal, state, local and foreign income taxes; and |
| (6) | This offering and the application of the estimated net proceeds from this offering as described under “Use of Proceeds.” |
The unaudited pro forma consolidated statements of income for the nine months ended September 30, 2020 and for the year ended December 31, 2019 give effect to the Pro Forma Transactions (as defined above) as if the Pro Forma Transactions had occurred or had become effective as of January 1, 2019. The unaudited pro forma consolidated balance sheet gives effect to the Pro Forma Transactions as if the Pro Forma Transactions had occurred or had become effective as of September 30, 2020.
Our historical consolidated financial information has been derived from our consolidated financial statements and accompanying notes to the consolidated financial statements included elsewhere in this prospectus. Maravai LifeSciences Holdings, Inc. was formed on August 25, 2020 and will have no material assets or results of operations until the completion of this offering. Therefore, Maravai LifeSciences Holdings, Inc.’s historical financial information is not included in the unaudited pro forma consolidated financial information.
The unaudited pro forma consolidated financial information has been prepared on the basis that we will be taxed as a corporation for U.S. federal and state income tax purposes and, accordingly, will become a taxpaying entity subject to U.S. federal, state and foreign income taxes. The presentation of the unaudited pro forma consolidated financial information is prepared in conformity with Article 11 of Regulation S-X and is based on currently available information and certain estimates and assumptions. The unaudited pro forma consolidated financial information has been adjusted to give effect to events that are (i) directly attributable to the transactions, (ii) factually supportable and (iii) with respect to the statements of operations, expected to have a continuing impact on the results of operations. See the accompanying notes to the Unaudited Consolidated Pro Forma Financial Information for a discussion of assumptions made.
The unaudited pro forma consolidated financial information is not necessarily indicative of financial results that would have been attained had the Pro Forma Transactions occurred on the dates indicated above or that could be achieved in the future. The unaudited pro forma consolidated financial information also does not give effect to the potential impact of any anticipated synergies, operating efficiencies or cost savings that may result from the Pro Forma Transactions or any integration costs that result from the Organizational Transactions or any costs that do not have a continuing impact. Future results may vary significantly from the results reflected in the unaudited pro forma consolidated statements of income and should not be relied on as an indication of our results after the consummation of this offering and the other transactions contemplated by such unaudited pro forma
87
consolidated financial information. However, management believes that the assumptions provide a reasonable basis for presenting the significant effects of the Pro Forma Transactions as contemplated and that the pro forma adjustments give appropriate effect to those assumptions and are properly applied in the unaudited pro forma consolidated financial information.
As a public company, we will be implementing additional procedures and processes for the purpose of addressing the standards and requirements applicable to public companies. We expect to incur additional annual expenses related to these steps and, among other things, additional directors’ and officers’ liability insurance, director fees, fees to comply with the reporting requirements of the SEC, transfer agent fees, hiring of additional accounting, legal and administrative personnel, increased auditing and legal fees and similar expenses. We have not included any pro forma adjustments relating to these costs.
For purposes of the unaudited pro forma consolidated financial information, we have assumed that we will issue 50,000,000 shares of Class A common stock at a price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus, and, as a result, immediately following the completion of this offering, the ownership percentage represented by LLC Units not held by us will be 68%, and the net income attributable to LLC Units not held by us will accordingly represent 68% of our net income or loss. Except as otherwise indicated, the unaudited pro forma consolidated financial information presented assumes no exercise by the underwriters of their option to purchase additional shares of Class A common stock.
As described in greater detail under “Certain Relationships and Related Party Transactions—Tax Receivable Agreement,” in connection with the consummation of this offering, we will enter into the Tax Receivable Agreement with MLSH 1 and MLSH 2 that will provide for the payment by Maravai LifeSciences Holdings, Inc. to MLSH 1 and MLSH 2, collectively, of 85% of the amount of cash savings, if any, in U.S. federal, state and local income taxes (computed using simplifying assumptions to address the impact of state and local taxes) we actually realize (or under certain circumstances are deemed to realize in the case of an early termination payment by us, a change in control or a material breach by us of our obligations under the Tax Receivable Agreement, as discussed below) as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement.
We expect to benefit from the remaining 15% of cash savings, if any, that we realize. As a result of the Organizational Transactions and the purchase of currently outstanding LLC Units from MLSH 1 with a portion of the net proceeds from this offering, we are recording a liability under the Tax Receivable Agreement of $279.8 million as described in more detail below. Due to the uncertainty in the amount and timing of future exchanges of LLC Units by LLC Unitholders and purchases of LLC Units from LLC Unitholders, the unaudited pro forma consolidated financial information assumes that no future exchanges or purchases of LLC Units have occurred and therefore no increases in tax basis in the Topco LLC assets or other tax benefits that may be realized thereunder have been assumed in the unaudited pro forma consolidated financial information. However, if all of the LLC Unitholders were to exchange or sell us all of their LLC Units, we would recognize a deferred tax asset of approximately $1,548.5 million and a liability under the Tax Receivable Agreement of approximately $1,374.8 million, assuming: (i) all exchanges or purchases occurred on the same day; (ii) a price of $25.50 per share, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus; (iii) a corporate tax rate of 24.19% and 24.01% as of December 31, 2019 and September 30, 2020; (iv) that we will have sufficient taxable income to fully utilize the tax benefits and (v) no material changes in tax law. These amounts are estimates and have been prepared for informational purposes only. The actual amount of deferred tax assets and related liabilities that we will recognize will differ based on, among other things, the timing of the exchanges, the price per share of our Class A common stock at the time of the exchange, and the tax rates then in effect.
88
For each 5% increase in the amount of LLC Units exchanged by or purchased from MLSH 1 and MLSH 2, our deferred tax asset would increase by approximately $13.6 million and the related liability would increase by approximately $12.1 million, and for each 5% decrease in the amount of LLC Units exchanged by or purchased from MLSH 1 and MLSH 2, our deferred tax asset would decrease by approximately $13.8 million and the related liability would decrease by approximately $13.3 million, assuming that the price per share and corporate tax rate remain the same. For each $1.00 increase (decrease) in the assumed share price of $25.50 per share, our deferred tax asset would increase (decrease) by approximately $10.9 million and the related liability would increase (decrease) by approximately $9.3 million, assuming that the number of LLC Units exchanged by or purchased from MLSH 1 and MLSH 2 (or their transferees of LLC Units and other assignees) and the corporate tax rate remain the same. These amounts are estimates and have been prepared for informational purposes only. The actual amount of deferred tax assets and liability under the Tax Receivable Agreement that we will recognize will differ based on, among other things, the timing of the exchanges and purchases, the price of our shares of Class A common stock at the time of the exchange or purchase, and the tax rates then in effect.
The unaudited pro forma consolidated financial information should be read together with “Organizational Structure,” “ Use of Proceeds,” “Capitalization,” “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Certain Relationships and Related Party Transactions” and the audited annual consolidated financial statements of Topco LLC and related notes thereto as well as the unaudited interim consolidated financial statements of Topco LLC and related notes thereto, each of which are included elsewhere in this prospectus.
89
UNAUDITED CONSOLIDATED PRO FORMA BALANCE SHEET
AS OF SEPTEMBER 30, 2020
| (In thousands, except per share data) |
Topco LLC As Reported |
Debt Refinancing and MLSC Minority Interest Buyout Adjustments |
Topco LLC As Adjusted |
Organizational Transaction Adjustments |
Maravai LifeSciences Holdings, Inc. Pro Forma |
|||||||||||||||||||||||
| Assets |
||||||||||||||||||||||||||||
| Current assets: |
||||||||||||||||||||||||||||
| Cash |
$ | 124,882 | $ | 510 | (1) | $ | 125,392 | $ | 46,265 | (9)(10)(11) | $ | 171,657 | ||||||||||||||||
| Accounts receivable, net |
73,301 | — | 73,301 | — | 73,301 | |||||||||||||||||||||||
| Inventory |
26,586 | — | 26,586 | — | 26,586 | |||||||||||||||||||||||
| Prepaid expenses and other current assets |
9,352 | 9,352 | (2,574 | ) | (12) | 6,778 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total current assets |
234,121 | 510 | 234,631 | 43,691 | 278,322 | |||||||||||||||||||||||
| Property and equipment, net |
96,089 | — | 96,089 | — | 96,089 | |||||||||||||||||||||||
| Goodwill |
224,275 | — | 224,275 | — | 224,275 | |||||||||||||||||||||||
| Intangible assets, net |
182,820 | — | 182,820 | — | 182,820 | |||||||||||||||||||||||
| Deferred tax asset |
— | — | — | 307,346 | (13)(14) | 307,346 | ||||||||||||||||||||||
| Other assets |
807 | 3,372 | (6) | 4,179 | — | 4,179 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total assets |
$ | 738,112 | $ | 3,882 | $ | 741,994 | $ | 351,037 | $ | 1,093,031 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Liabilities and member’s/stockholders’ equity |
||||||||||||||||||||||||||||
| Current liabilities: |
||||||||||||||||||||||||||||
| Accounts payable |
$ | 7,796 | $ | — | $ | 7,796 | $ | — | $ | 7,796 | ||||||||||||||||||
| Accrued expenses |
33,683 | (3,004 | ) | (2) | 30,679 | 14,298 | (12) | 44,977 | ||||||||||||||||||||
| Repurchase liability for incentive units |
9,140 | (9,140 | ) | (3) | — | — | — | |||||||||||||||||||||
| Redeemable noncontrolling interest |
166,427 | (120,005 | ) | (4) | 46,422 | (46,422 | ) | (15) | — | |||||||||||||||||||
| Deferred revenue |
60,674 | — | 60,674 | — | 60,674 | |||||||||||||||||||||||
| Other current liabilities |
231 | — | 231 | 2,803 | (16) | 3,034 | ||||||||||||||||||||||
| Current portion of long-term debt |
2,500 | 2,000 | (5) | 4,500 | — | 4,500 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total current liabilities |
280,451 | (130,149 | ) | 150,302 | (29,321 | ) | 120,981 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Long-term debt, less current portion |
349,204 | 230,896 | (6) | 580,100 | (50,000 | ) | (11) | 530,100 | ||||||||||||||||||||
| Deferred tax liabilities |
13,422 | — | 13,422 | — | 13,422 | |||||||||||||||||||||||
| Facility financing obligations, less current portion |
56,440 | — | 56,440 | — | 56,440 | |||||||||||||||||||||||
| TRA liability |
— | — | — | 279,796 | (14) | 279,796 | ||||||||||||||||||||||
| Other long-term liabilities |
2,601 | — | 2,601 | — | 2,601 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total liabilities |
$ | 702,118 | $ | 100,747 | $ | 802,865 | $ | 200,475 | $ | 1,003,340 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
90
| (In thousands, except per share data) |
Topco LLC As Reported |
Debt Refinancing and MLSC Minority Interest Buyout Adjustments |
Topco LLC As Adjusted |
Organizational Transaction Adjustments |
Maravai LifeSciences Holdings, Inc. Pro Forma |
|||||||||||||||||||||||
| Member’s/stockholders’ equity: |
||||||||||||||||||||||||||||
| Class A common stock, par value 0.01 per share 500,000,000 shares authorized; 81,379,818 shares issued and outstanding |
— | — | 814 | (9)(17) | 814 | |||||||||||||||||||||||
| Class B common stock, par value 0.01 per share 300,000,000 shares authorized; 176,458,692 shares issued and outstanding |
— | — | 1,765 | (10) | 1,765 | |||||||||||||||||||||||
| Additional paid in capital |
— | — | 105,298 | (21) | 105,298 | |||||||||||||||||||||||
| Contributed capital, 253,916,941 units authorized, issued and outstanding |
14,777 | (14,777 | ) | (7) | — | — | (15)(18) | — | ||||||||||||||||||||
| Retained earnings (accumulated deficit) |
21,381 | (82,087 | ) | (7)(8) | (60,706 | ) | (667 | ) | (19)(20) | (61,373 | ) | |||||||||||||||||
| Accumulated other comprehensive loss |
(164 | ) | — | (164 | ) | 112 | (20) | (52 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total member’s/stockholders’ equity (deficit) |
35,994 | (96,864 | ) | (60,870 | ) | 107,322 | 46,452 | |||||||||||||||||||||
| Noncontrolling interests |
— | — | — | 43,239 | (20) | 43,239 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total member’s/stockholders’ equity (deficit) |
35,994 | (96,864 | ) | (60,870 | ) | 150,561 | 89,691 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total liabilities and member’s /stockholders’ equity |
$ | 738,112 | $ | 3,883 | $ | 741,995 | $ | 351,036 | $ | 1,093,031 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
91
NOTES TO UNAUDITED CONSOLIDATED PRO FORMA BALANCE SHEET
| (1) | Represents the net adjustment to cash to reflect the net cash proceeds of $594.0 million ($600.0 million less $6.0 million of original issue discount) received from the issuance of the New Term Loan and the payment of (i) $360.0 million to repay historical long term debt as discussed in note (6), (ii) $129.1 million in cash consideration to repurchase a) outstanding time-based MLSC Holdings, LLC incentive units for $9.1 million and b) MLSC Holdings, LLC Class B preferred units and common units for $120.0 million, (iii) an $88.6 million distribution to MLSH 1, (iv) $12.8 million related to the payment of debt issuance costs and (v) $3.0 million related to the payment of accrued interest on the historical First Lien Term Loan and Second Lien Term Loan. |
| (2) | Represents the adjustment to accrued expenses to reflect the payment of $3.0 million in accrued interest associated with repayment of the historical First Lien Term Loan and Second Lien Term Loan. |
| (3) | Represents the repurchase and settlement of all outstanding time-based MLSC Holdings, LLC incentive units for $9.1 million in cash from the investors of MLSC Holdings, LLC, the parent of Cygnus Technologies. |
| (4) | Represents the repurchase and settlement of 43,264 MLSC Holdings, LLC Class B preferred units and 18,387,206 common units for $120.0 million in cash from the investors of MLSC Holdings, LLC, the parent of Cygnus Technologies. |
| (5) | Represents the net adjustment to the current portion of long-term debt to reflect (i) the repayment of $2.5 million of the current portion of the historical first lien and second lien term loans and (ii) the current portion of the New Term Loan of $4.5 million. |
| (6) | Represents the net adjustment to total long-term debt associated with the debt refinancing (in millions): |
| Issuance of the New Term Loan, net of current portion of $4.5 million |
$ | 595.5 | ||
| Original issuance discount of New Term Loan |
(6.0 | ) | ||
| Debt issuance costs of New Term Loan |
(9.4 | ) | ||
| Repayment of historical First Lien Term Loan, net of current portion of $2.5 million |
(242.5 | ) | ||
| Repayment of Second Lien Term Loan |
(100.0 | ) | ||
| Repayment of Revolving Credit Facility |
(15.0 | ) | ||
| Write-off of unamortized debt issuance costs associated with extinguishment of outstanding debt |
8.3 | |||
|
|
|
|||
| Net adjustment to long-term debt |
$ | 230.9 | ||
|
|
|
Additionally, an adjustment of $3.4 million was made to other assets to reflect the debt issuance costs related to the New Revolving Credit Facility.
| (7) | Represents the adjustment to reduce contributed capital to zero to reflect $14.8 million of the total $88.6 million distribution paid to MLSH 1 in connection with the debt refinancing. The remainder of the distribution, $73.8 million, has been reflected as an increase to accumulated deficit as discussed in note (8). |
| (8) | Represents the adjustment to retained earnings (accumulated deficit) to reflect (i) the write off of unamortized debt issuance costs of $8.3 million in connection with the debt repayments discussed in note (6) and (ii) $73.8 million of the distribution paid to MLSH 1 discussed in note (7). |
| (9) | We estimate that the proceeds to us from this offering will be approximately $1,204.9 million (or $1,385.6 million if the underwriters exercise in full their option to purchase additional shares of Class A common stock), based on an assumed initial public offering price of $25.50 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, after deducting $70.1 million of assumed underwriting discounts and commissions. We intend to use the net proceeds from this offering to (i) acquire 3,921,569 newly issued LLC Units from Topco for $94.5 million, (ii) acquire 39,121,430 currently-outstanding LLC Units from MLSH 1 for $942.7 million and (iii) pay MLSH 2 |
92
| $167.6 million as consideration for the Blocker Mergers. In turn, Topco intends to apply the proceeds it receives from us to {i) repay a portion of the New Term Loan discussed in note (11), (ii) pay expenses incurred in connection with this offering discussed in note (12) and (iii) for general corporate purposes. For more information, see “Use of Proceeds.” |
| (10) | Reflects the issuance of Class B common stock to LLC Unitholders, on a one-to-one basis with the number of LLC Units they own, in exchange for cash consideration of $1.8 million equal to the par value of the Class B common stock issued, as described in greater detail under “Organizational Structure.” |
| (11) | Reflects the partial repayment of the New Term Loan using $50.0 million of proceeds received from the offering. |
| (12) | We are deferring certain costs associated with this offering. These costs primarily represent legal, accounting and other costs directly associated with this offering. As of September 30, 2020, $2.6 million of these costs were recorded to prepaid expenses and other current assets, and an additional $3.2 million of capitalizable costs were incurred subsequent to September 30, 2020. Upon completion of this offering, these deferred costs will be charged against the proceeds from this offering with a corresponding reduction to additional paid-in capital as discussed in note (21). After September 30, 2020, the Company incurred an additional $0.9 million of costs associated with this offering that were not eligible for capitalization. These costs were expensed as incurred and were recorded to accrued expenses and retained earnings. |
| (13) | Maravai LifeSciences Holdings, Inc. is subject to U.S. federal, state, local and foreign income taxes and will file consolidated income tax returns for U.S. federal and certain st |
| ate, local and foreign jurisdictions. This adjustment reflects the recognition of deferred taxes in connection with the Organizational Transactions assuming the federal rates currently in effect and the highest statutory rates apportioned to each state, local and foreign jurisdiction. |
We have recorded a pro forma deferred tax asset adjustment of $307.3 million. The deferred tax asset includes (i) $251.9 million related to temporary differences in the book basis as compared to the tax basis of our Company’s investment in Topco LLC and (ii) $57.8 million related to tax benefits from future deductions attributable to payments under the tax receivable agreement as described further in note (14). A valuation allowance of $2.4 million has been recorded for those deferred tax assets which Maravai LifeSciences Holdings, Inc. has determined are not more likely than not to be realized. Maravai LifeSciences Holdings, Inc. has determined it is more likely than not the remaining $307.3 million of deferred tax assets will result in ordinary income tax deductions that will be realized based on projections of future taxable income. Maravai LifeSciences Holdings, Inc. will continue to assess all positive and negative evidence and will adjust the valuation allowance to the extent it is more likely than not its assessment changes.
| (14) | Prior to the completion of this offering, we will enter into a tax receivable agreement with MLSH 1 and MLSH 2. The agreement provides for the payment to MLSH 1 and MLSH 2 of 85% of the benefits, if any, that we realize as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement. The Tax Receivable Agreement will be accounted for as a contingent liability due to related parties, with amounts accrued when considered probable and reasonably estimable. We will record a $279.8 million liability based on the Company’s estimate of the aggregate amount that it will pay to MLSH 1 and MLSH 2 under the tax receivable agreement as a result of the Organizational Transactions. As mentioned in note (13) above, we will record an increase of $57.8 million in deferred tax assets related to tax benefits from future deductions attributable to payments under the tax receivable agreement as a result of the Organizational Transactions. Additionally, we will record a decrease to additional paid-in capital of $222.0 million, which is equal to the difference between the increase in deferred tax assets and the increase in liabilities due to existing owners under the tax receivable agreement as a result of the Organizational Transactions. |
93
No adjustment has been made to reflect future exchanges by LLC Unitholders (or their transferees of LLC Units or other assignees) of LLC Units for cash or shares of our Class A common stock, as applicable.
| (15) | Represents the contribution to MLSH of the remaining 16,736 MLSC Holdings, LLC Class B preferred units and 7,112,794 common units from the investors of MLSC Holdings, LLC, the parent of Cygnus Technologies in exchange for a variable number of common units of MLSH 1 with a fixed total value of $46.4 million. |
| (16) | Reflects the conversion of MockV Solutions, Inc. to a limited liability company, which is expected to be treated as a taxable liquidation resulting in an increase of $2.8 million in taxes payable. |
| (17) | As part of the Organizational Transactions, the Blocker Entities will merge with and into Maravai LifeSciences Holdings, Inc. with MLSH 2 receiving as consideration 31,379,818 shares of Class A common stock and $167.6 million of proceeds from the offering, as discussed in note (9). As a result of the Blocker Mergers, Maravai LifeSciences Holdings, Inc. will obtain 38,336,819 LLC Units of Topco LLC. |
| (18) | Represents an adjustment of $46.4 million to reflect the reclassification of contributed capital to additional paid-in capital after giving effect to the distribution paid to MLSH 1 as part of the debt refinancing discussed in note (7), and the contribution to MLSH 1 of the remaining MLSC Holdings, LLC Class B preferred units and common units from the investors of MLSC Holdings, LLC, the parent of Cygnus Technologies as described in note (15) above. |
| (19) | Represents an adjustment of $3.5 million to retained earnings and additional-paid-in-capital to reflect the vesting of 277,500 MLSH 1 performance-based incentive units whose performance condition is satisfied upon the completion of this offering. |
| (20) | As a result of the Organizational Transactions, the limited liability company agreement of Topco LLC will be amended and restated to, among other things, designate Maravai LifeSciences Holdings, Inc. as the sole managing member of Maravai Topco Holdings, LLC. As sole managing member, Maravai LifeSciences Holdings, Inc. will exclusively operate and control the business and affairs of Maravai Topco Holdings, LLC. The LLC Units owned by LLC Unitholders will be considered noncontrolling interests in the consolidated financial statements of Maravai LifeSciences Holdings, Inc. The adjustments to (i) non-controlling interest of $43.2 million, (ii) additional paid in capital of $47.1 million, (iii) retained earnings of $3.8 million, and (iv) accumulated other comprehensive income of $0.1 million reflect the proportional interest in the pro forma consolidated total equity of Maravai Topco Holdings, LLC owned by MLSH 1. |
| (21) | The following table is a reconciliation of the adjustments impacting additional paid-in-capital (in millions): |
| Net proceeds from offering of Class A common stock |
$ | 1,204.4 | (9) | |||||
| Purchase of LLC Units from MLSH 1 |
(942.7 | ) | (9) | |||||
| Reclassification of deferred costs incurred in this offering to additional paid-in capital |
(16.0 | ) | (12) | |||||
| Net adjustment from recognition of deferred tax assets and payable to related parties pursuant to the Tax Receivable Agreement |
24.7 | (13)(14)(16) | ||||||
| Acquisition of Blocker Mergers |
(168.0 | ) | (17) | |||||
| Reclassification of contributed capital |
46.4 | (18) | ||||||
| Adjustment for vested incentive units |
3.5 | (19) | ||||||
| Adjustment for non-controlling interest |
(47.1 | ) | (20) | |||||
|
|
|
|||||||
| Net additional paid-in capital pro forma adjustment |
$ | 105.3 | ||||||
|
|
|
94
UNAUDITED CONSOLIDATED PRO FORMA STATEMENT OF INCOME FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2020
| (In thousands, except per share data) |
Topco LLC As Reported |
Debt Refinancing and MLSC Minority Interest Buyout Adjustments |
Topco LLC As Adjusted |
Organizational Transaction Adjustments |
Maravai LifeSciences Holdings, Inc. Pro Forma |
|||||||||||||||||||||||
| Revenue |
$ | 185,745 | — | $ | 185,745 | — | $ | 185,745 | ||||||||||||||||||||
| Operating Expenses: |
||||||||||||||||||||||||||||
| Cost of revenue |
56,254 | — | 56,254 | — | 56,254 | |||||||||||||||||||||||
| Research and development |
7,212 | — | 7,212 | — | 7,212 | |||||||||||||||||||||||
| Selling, general and administrative |
52,624 | (1,483 | ) | (1) | 51,141 | 7,188 | (4) | 58,329 | ||||||||||||||||||||
| Gain on sale and leaseback transaction |
(19,002 | ) | — | (19,002 | ) | — | (19,002 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total operating expenses |
97,088 | (1,483 | ) | 95,605 | 7,188 | 102,793 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Income from operations |
88,657 | 1,483 | 90,140 | (7,188 | ) | 82,952 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||||||
| Interest expense |
(21,934 | ) | (3,224 | ) | (2) | (25,158 | ) | 2,178 | (5) | (22,980 | ) | |||||||||||||||||
| Other income |
132 | 132 | 132 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Income before income taxes |
66,855 | (1,741 | ) | 65,114 | (5,010 | ) | 60,104 | |||||||||||||||||||||
| Income tax expense |
2,511 | 2,511 | 3,504 | (6) | 6,015 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Net income |
64,344 | (1,741 | ) | 62,603 | (8,514 | ) | 54,089 | |||||||||||||||||||||
| Net income (loss) attributable to noncontrolling interests |
582 | (420 | ) | (3) | 162 | 39,253 | (7)(8) | 39,415 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Net income (loss) attributable to Maravai LifeSciences Holdings, Inc. |
$ | 63,762 | $ | (1,321 | ) | $ | 62,441 | $ | (47,767 | ) | $ | 14,674 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Pro Forma Earnings Per Share |
||||||||||||||||||||||||||||
| Basic |
(9) | $ | 0.18 | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Diluted |
(9) | $ | 0.18 | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Pro Forma Number of Shares Used in Computing EPS |
||||||||||||||||||||||||||||
| Basic |
(9) | 84,465,072 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Diluted |
(9) | 84,499,582 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
95
UNAUDITED CONSOLIDATED PRO FORMA STATEMENT OF INCOME
FOR THE YEAR ENDED DECEMBER 31, 2019
| (In thousands, except per share data) |
Topco LLC As Reported |
Debt Refinancing and MLSC Minority Interest Buyout Adjustments |
Topco LLC As Adjusted |
Organizational Transaction Adjustments |
Maravai LifeSciences Holdings, Inc. Pro Forma |
|||||||||||||||||||||||
| Revenues |
$ | 143,140 | — | $ | 143,140 | — | $ | 143,140 | ||||||||||||||||||||
| Operating Expenses: |
||||||||||||||||||||||||||||
| Cost of revenue |
66,849 | — | 66,849 | — | 66,849 | |||||||||||||||||||||||
| Research and development |
3,627 | — | 3,627 | — | 3,627 | |||||||||||||||||||||||
| Selling, general and administrative |
48,354 | (352 | ) | (1) | 48,002 | 9,584 | (4) | 57,586 | ||||||||||||||||||||
| Change in estimated fair value of contingent consideration |
322 | — | 322 | — | 322 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total operating expenses |
119,152 | (352 | ) | 118,800 | 9,584 | 128,384 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Income from operations |
23,988 | 352 | 24,340 | (9,584 | ) | 14,756 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||||||
| Interest expense |
(29,959 | ) | (1,942 | ) | (2) | (31,901 | ) | 2,736 | (5) | (29,165 | ) | |||||||||||||||||
| Other income |
118 | — | 118 | — | 118 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Loss before income taxes |
(5,853 | ) | (1,590 | ) | (7,443 | ) | (6,848 | ) | (14,291 | ) | ||||||||||||||||||
| Income tax (benefit) expense |
(652 | ) | — | (652 | ) | (907 | ) | (6) | (1,559 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Net income (loss) |
(5,201 | ) | (1,590 | ) | (6,791 | ) | (5,941 | ) | (12,732 | ) | ||||||||||||||||||
| Net income (loss) attributable to noncontrolling interests |
(731 | ) | 527 | (3) | (204 | ) | (9,131 | ) | (7)(8) | (9,335 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Net income (loss) attributable to Maravai LifeSciences Holdings, Inc. |
$ | (4,470 | ) | $ | (2,117 | ) | $ | (6,587 | ) | $ | 3,190 | $ | (3,397 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Pro Forma Earnings Per Share |
||||||||||||||||||||||||||||
| Basic |
(9) | $ | (0.04 | ) | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Diluted |
(9) | $ | (0.04 | ) | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Pro Forma Number of Shares Used in Computing EPS |
||||||||||||||||||||||||||||
| Basic |
(9) | 84,465,072 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Diluted |
(9) | 84,465,072 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
96
NOTES TO UNAUDITED CONSOLIDATED PRO FORMA STATEMENTS OF INCOME
| (1) | Represents the adjustment to remove unit-based compensation expense of $1.5 million and $0.4 million for the nine months ended September 30, 2020 and year ended December 31, 2019, respectively, that was previously recognized for the MLSC Holdings, LLC incentive units that were repurchased from the investors of MLSC Holdings, LLC, the parent of Cygnus Technologies. |
| (2) | Represents the net adjustment to interest expense associated with the debt refinancing (in millions): |
| Nine months ended September 30, 2020 |
Year ended December 31, 2019 |
|||||||
| Interest expense—New Term Loan |
$ | 25.2 | $ | 31.9 | ||||
| Interest expense—First Lien Term Loan and Second Lien Term Loan |
(21.9 | ) | (30.0 | ) | ||||
|
|
|
|
|
|||||
| Net adjustments to interest expense |
$ | 3.2 | $ | 1.9 | ||||
|
|
|
|
|
|||||
The interest rate on the New Term Loan reflects a LIBOR floor of 2% plus a margin of 3.25%. For each 0.125% increase in the interest rate on the New Term Loan, interest expense would increase by approximately $0.6 million and $0.7 million in the nine months ended September 30, 2020 and year ended December 31, 2019, respectively.
| (3) | Represents the $0.4 million reduction to net income attributable to noncontrolling interest for the nine months ended September 30, 2020 and the $0.5 million reduction to net loss attributable to noncontrolling interests for the year ended December 31, 2019 to reflect the repurchase and settlement of the outstanding 43,264 MLSC Holdings, LLC Class B preferred units and 18,387,206 common units from the investors of MLSC Holdings, LLC, the parent of Cygnus Technologies. |
| (4) | Reflects the recognition of compensation expense totaling $7.2 million for the nine months ended September 30, 2020 and $9.6 million for the year ended December 31, 2019 related to (i) the granting of 1,584,400 stock options to employees to purchase Class A common stock and (ii) the issuance of 75,294 restricted stock units to our non-employee directors in connection with the offering. The foregoing amounts are based on an assumed initial public offering price of $25.50 per share (which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus). The stock options are expected to vest one-fourth on each of the first four anniversaries of the grant date and the restricted stock units will vest ratably over three years. |
| (5) | Represents a reduction to interest expense associated with the partial repayment of the New Term Loan using $50.0 million of proceeds received from the Offering (in millions). |
| Nine months ended September 30, 2020 |
Twelve months ended December 31, 2019 |
|||||||
| Interest expense—Partially repaid New Term Loan |
$ | 23.0 | $ | 29.2 | ||||
| Interest expense—New Term Loan |
(25.2 | ) | (31.9 | ) | ||||
|
|
|
|
|
|||||
| Reduction to interest expense from partial repayment |
$ | (2.2 | ) | $ | (2.7 | ) | ||
|
|
|
|
|
|||||
| (6) | Following the Organizational Transactions and offering, Maravai LifeSciences Holdings, Inc. will be subject to U.S. federal income taxes, in addition to state, local and foreign taxes. As a result, the pro forma statements of income reflect an adjustment to our provision for corporate income taxes to reflect a pro forma tax rate, which includes a provision for U.S. federal income taxes and assumes the highest statutory rates apportioned to each state, local and foreign jurisdiction. Topco LLC has been, and will continue to be, treated as a partnership for U.S. federal and state income tax purposes. As such, Topco LLC’s profits and losses will flow through to its partners, including Maravai LifeSciences Holdings, Inc., and are generally not subject to tax at the Topco LLC level. |
97
The pro forma adjustments for income tax expense represent tax expense (benefit) on income that will be taxable in jurisdictions after our Organizational Transactions that previously had not been taxable. The adjustment is calculated as pro forma Topco LLC pass-through net income multiplied by the ownership percentage of the controlling interest and multiplied by the pro forma corporate tax rate.
| Nine months ended September 30, 2020 |
Twelve months ended December 31, 2019 |
|||||||
| Pro forma Topco LLC net income (loss) |
$ | 57.6 | $ | (13.6 | ) | |||
| Pro forma Topco LLC net income (loss) taxed at the corporate rate |
(11.4 | ) | 1.8 | |||||
|
|
|
|
|
|||||
| Pro forma Topco LLC pass-through net income (loss) |
$ | 46.2 | $ | (11.9 | ) | |||
| Ownership percentage of the controlling interest |
31.56 | % | 31.56 | % | ||||
|
|
|
|
|
|||||
| Pro forma taxable income (loss) attributable to the controlling interest |
14.6 | (3.7 | ) | |||||
| Pro forma corporate tax rate |
24.01 | % | 24.19 | % | ||||
|
|
|
|
|
|||||
| Pro forma income tax expense adjustment |
$ | 3.5 | $ | (0.9 | ) | |||
|
|
|
|
|
|||||
| (7) | Represents the $0.2 million reduction to net income attributable to noncontrolling interest for the nine months ended September 30, 2020 and the $0.2 million reduction to net loss attributable to noncontrolling interests for the year ended December 31, 2019 to reflect the exchange of the outstanding 16,736 MLSC Holdings, LLC Class B preferred units and 7,112,794 common units into a variable number of common units of MLSH 1 with a fixed total value of approximately $46.4 million. |
| (8) | Following the Organizational Transactions, Maravai LifeSciences Holdings, Inc. will become the sole managing member of Topco LLC, and upon consummation of this offering, Maravai LifeSciences Holdings, Inc. will initially own approximately 32% of the economic interest in Topco LLC but will have 100% of the voting power and control the management of Topco LLC. The ownership percentage held by the noncontrolling interest, MLSH 1, will be approximately 68%. Net income attributable to the noncontrolling interest will represent approximately 68% of net income. |
| (9) | The weighted average number of shares underlying the basic earnings per share calculation reflects only the 81,379,818 shares of Class A common stock outstanding after the offering as they are the only outstanding shares which participate in distributions or dividends by Maravai LifeSciences Holdings, Inc. The net proceeds from the sale of 50,000,000 shares of Class A common stock in the IPO will be used to (i) acquire 3,921,569 newly issued LLC Units from Topco, (ii) acquire 39,121,430 currently-outstanding LLC Units from MLSH 1, and (iii) pay MLSH 2 $167.6 million as consideration for the Blocker Mergers. Pro forma diluted earnings per share is computed by adjusting pro forma net income attributable to Maravai LifeSciences Holdings, Inc. and the weighted average shares of Class A common stock outstanding to give effect to potentially dilutive securities that qualify as participating securities using the treasury stock method, as applicable. Shares of Class B common stock are not participating securities and therefore are not included in the calculation of pro forma basic earnings per share. LLC Units, together with an equal number of shares of Class B common stock, may be exchanged, at our option, for shares of our Class A common stock or for cash. After evaluating the potential dilutive effect under the if-converted method, the outstanding LLC Units for the assumed exchange of non-controlling interests were determined to be antidilutive and thus were excluded in the computation of diluted earnings per share. The diluted weighted average share calculation assumes that certain equity awards were issued and outstanding at the beginning of the period. The following table sets forth a reconciliation of the numerators and denominators used to compute pro forma basic and diluted earnings per share. |
98
| Nine Months Ended September 30, 2020 |
Twelve Months Ended December 31, 2019 |
|||||||
| Earnings (loss) per share of common stock |
||||||||
| Numerator: |
||||||||
| Net income (loss) attributable to Maravai LifeSciences Holdings, Inc.’s shareholders (basic) |
$ | 15,049 | $ | (3,509 | ) | |||
| Reallocation of net income (loss) assuming issuance of LLC Units to the Company with respect to restricted stock units |
6 | — | ||||||
|
|
|
|
|
|||||
| Net income (loss) attributable to Maravai LifeSciences Holdings, Inc.’s shareholders (diluted) |
$ | 15,056 | $ | (3,509 | ) | |||
|
|
|
|
|
|||||
| Denominator: |
||||||||
| Weighted average of shares of common stock outstanding |
81,379,818 | 81,379,818 | ||||||
| Assumed shares sold in this offering sufficient to pay dividends in excess of current year earnings |
1,124,470 | 1,124,470 | ||||||
| Assumed shares sold in this offering sufficient to partially repay debt |
1,960,784 | 1,960,784 | ||||||
| Weighted average of shares of common stock outstanding (basic) |
84,465,072 | 84,465,072 | ||||||
| Incremental common shares attributable to dilutive instruments |
34,510 | — | ||||||
|
|
|
|
|
|||||
| Weighted average of shares of common stock outstanding (diluted) |
84,499,582 | 84,465,072 | ||||||
|
|
|
|
|
|||||
| Basic earnings (loss) per share |
$ | 0.18 | $ | (0.04 | ) | |||
|
|
|
|
|
|||||
| Diluted earnings (loss) per share |
$ | 0.18 | $ | (0.04 | ) | |||
|
|
|
|
|
|||||
99
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of financial condition and results of operations together with the section titled “Selected Consolidated Financial Data” and our audited consolidated financial statements and unaudited interim condensed consolidated financial statements and related notes included elsewhere in this prospectus. This discussion and analysis reflects our historical results of operations and financial position, and, except as otherwise indicated below, does not give effect to the Organizational Transactions or to the completion of this offering. See “Organizational Structure.” This discussion and other parts of this prospectus contain forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed in or implied by these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section titled “Risk Factors.” Please also see the section titled “Forward Looking Statements.”
The following discussion contains references to the years ended December 31, 2018 and 2019 and the nine months ended September 30, 2019 and 2020, which represent the consolidated and combined financial results of our predecessor Maravai Topco Holdings, LLC (“Topco LLC”) and subsidiaries for the years ended December 31, 2018 and 2019 and the nine months ended September 30, 2019 and 2020, respectively. Unless we state otherwise or the context otherwise requires, the terms “we,” “us,” “our,” and “Maravai” refer to and similar references refer: (1) on or following the consummation of the Organizational Transactions, including this offering, to Maravai LifeSciences Holdings, Inc. and its consolidated subsidiaries, including Topco LLC, and (2) prior to the consummation of the Organizational Transactions, including this offering, to Topco LLC and its consolidated subsidiaries.
Overview
We are a leading life sciences company providing critical products to enable the development of drug therapies, diagnostics, novel vaccines and support research on human diseases. Our more than 5,000 customers as of September 30, 2020 include the top 20 global biopharmaceutical companies ranked by research and development expenditures according to industry consultants, and many other emerging biopharmaceutical and life sciences research companies, as well as leading academic research institutions and in vitro diagnostics companies. Our products address the key phases of biopharmaceutical development and include complex nucleic acids for diagnostic and therapeutic applications, antibody-based products to detect impurities during the production of biopharmaceutical products, and products to detect the expression of proteins in tissues of various species.
We have and will continue to build a transformative life sciences products company by acquiring businesses and accelerating their growth through capital infusions and industry expertise. Biomedical innovation is dependent on a reliable supply of reagents in the fields of nucleic acid production, biologics safety testing and protein labeling. From inventive startups to the world’s leading biopharmaceutical, vaccine, diagnostics and gene and cell therapy companies, these customers turn to us to solve their complex discovery challenges and help them streamline and scale their supply chain needs beginning from research and development through clinical trials to commercialization.
100
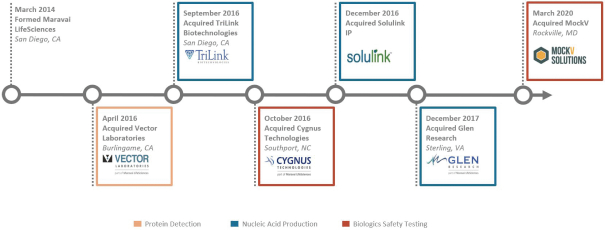
Our primary customers are biopharmaceutical companies who are pursuing novel research and product development programs. Our customers also include a range of government, academic and biotechnology institutions.
As of September 30, 2020, we employed a team of over 390 employees, approximately 17% of whom have advanced degrees. We primarily utilize a direct sales model for our sales to our customers in North America. Our international sales, primarily in Europe and Asia Pacific, are sold through a combination of third-party distributors as well as via a direct sales model. The percentage of our total revenue derived from customers in North America was 59.9% and 58.7% for the years ended December 31, 2018 and 2019, respectively, and 58.8% and 58.3% for the nine months ended September 30, 2019 and 2020, respectively.
We generated revenue of $123.8 million and $143.1 million for the years ended December 31, 2018 and 2019, respectively, and $107.2 million and $185.7 million for the nine months ended September 30, 2019 and 2020, respectively. Total revenue by segment was $60.0 million in nucleic acid production, $38.5 million in biologics safety testing and $25.3 million in protein detection for the year ended December 31, 2018, compared to $72.6 million, $44.4 million, and $26.1 million, respectively, for the year ended December 31, 2019. Total revenue by segment was $54.9 million in nucleic acid production, $32.8 million in biologics safety testing and $19.5 million in protein detection for the nine months ended September 30, 2019, compared to $129.6 million, $40.8 million and $16.4 million, respectively, for the nine months ended September 30, 2020.
Our research and development efforts are geared towards meeting our customers’ needs. We incurred research and development expenses of $4.5 million and $3.6 million for the years ended December 31, 2018 and 2019, respectively, and $2.6 million and $7.2 million for the nine months ended September 30, 2019 and 2020, respectively. We intend to continue to invest in research and development and new products and technologies to support our customers’ needs for the foreseeable future.
We focus a substantial portion of our resources supporting our core business segments. We are actively pursuing opportunities to expand our customer base both domestically and internationally by fostering strong relationships with both existing and new customers and distributors. Our management team has experience working with biopharmaceutical, vaccine, diagnostics and gene and cell therapy companies as well as academic and research scientists. We also intend to continue making investments in our overall infrastructure and business segments to support our growth. We incurred aggregate selling, general, and administrative expenses of $41.2 million and $48.4 million for the years ended December 31, 2018 and 2019, respectively, and $32.6 million and $52.6 million for the nine months ended September 30, 2019 and 2020, respectively.
101
Since our inception in 2016 through December 31, 2019, we have incurred net losses in each year. For the nine months ended September 30, 2019 and 2020, our net income was $0.2 million and $64.3 million, respectively. Our net losses were $16.9 million and $5.2 million for the years ended December 31, 2018 and 2019, respectively. We expect our expenses will increase substantially in connection with our ongoing activities, as we:
| • | attract, hire and retain qualified personnel; |
| • | invest in processes and infrastructure to enable manufacturing automation; |
| • | support research and development to introduce new products and services; |
| • | market and sell new and existing products and services; |
| • | protect and defend our intellectual property; |
| • | acquire businesses or technologies to support the growth of our business; and |
| • | function as a public company. |
Key Factors Affecting Our Results of Operations and Future Performance
We believe that our financial performance has been, and in the foreseeable future will continue to be, primarily driven by a number of factors as described below, each of which presents growth opportunities for our business. These factors also pose important challenges that we must successfully address in order to sustain our growth and improve our results of operations. Our ability to successfully address these challenges is subject to various risks and uncertainties, including those described under the heading “Risk Factors.”
Drug Development Pipelines
Our financial performance has largely been driven by our customers accelerating their drug development pipelines for cell, gene and RNA therapies. A key factor to our future success will be, our ability to provide good manufacturing practices (“GMP”) grade nucleic acids and associated pre-clinical and non-GMP compounds to these customers. Our GMP-grade nucleic acids are manufactured following certain voluntary GMP quality standards and customer specific requirements. We believe these products, including “GMP-grade” materials, are exempt from compliance with the current GMP regulations of the Food and Drug Administration (“FDA”). See “Business—Government Regulation.” The mRNA and gene editing therapeutics that many of our customers are developing are early in their lifecycle. We expect to see an increase in demand for our GMP-grade nucleic acids to the extent that our customers have success in their early-phase clinical trials of these therapeutics. New U.S. Food and Drug Administration (“FDA”) policies, and plans for maximizing the use of expedited programs, may advance the development of cell and gene therapies. Additionally, the COVID-19 pandemic has both fostered increased interest in mRNA as a therapeutic modality for this virus and directed significant resources to developing a base of knowledge for mRNA.
Demand for Outsourced GMP-grade RNA
We believe that growing numbers of RNA therapeutics companies expect to outsource production of pre-clinical and GMP-grade RNA to trusted business partners. Companies are often driven to outsource due to the complex nature of the manufacturing process, faster speeds to market, recent availability of high-quality contract development and manufacturing organizations (“CDMO”) partners, an influx of inexperienced and virtual biopharmaceutical companies, the need for redundancy of clinical and commercial supply and recent moves to onshore critical supply chains. We offer a number of products and services to meet this demand for outsourced pre-clinical and GMP-grade RNA.
102
Demand for Outsourced Biologics Safety Testing Products and Assay Development Services
We believe that many biopharmaceutical companies rely on outsourced providers for their biologics safety testing products and assay development needs. Once process development has been completed, biopharmaceutical companies avoid changing biologics safety testing products or providers for fear of affecting the regulatory approval pathway of their therapeutic products. This supports revenue growth for the biologics safety testing products that have been adopted by these companies. We also have long-standing relationships with many of our customers, which are bolstered by the regulatory demands on our customers and the “designed-in” nature of our products and services. A successful partnership with a customer related to the development of their drug leads to repeat business as customers become comfortable with our products. The drug approval process is smoother when regulatory bodies are familiar with an impurity detection product vendor and most biopharmaceutical customers are not willing to risk a regulatory issue related to biologics safety testing for their drug program on an unproven vendor. It is therefore critical to our success that our products and services be “designed-in” at the outset, especially to the most promising product candidates.
COVID-19 and Other Public Health Crises
In late 2019, COVID-19 surfaced in Wuhan, China. Since then, COVID-19 has spread to multiple other regions and countries, including the San Francisco Bay Area, where our protein detection business is located and the San Diego, California and Washington, D.C. areas, where our nucleic acid production business is located and the Wilmington, North Carolina area, where our biologics safety testing products business is located. The COVID-19 pandemic is evolving, and to date has led to the implementation of various responses, including government imposed shelter-in-place orders, quarantines, travel restrictions and other public health safety measures, as well as reported adverse impacts on healthcare resources, facilities and providers, in California, across the United States and in many other countries throughout the world. In response to the spread of COVID-19, and in accordance with direction from state and local government authorities, we have restricted access to our facilities mostly to personnel and third parties who must perform critical activities that must be completed on-site, limited the number of such personnel that can be present at our facilities at any one time, and requested that some of our personnel work remotely. In the event that government authorities were to further modify current restrictions, our employees conducting research and development, or manufacturing activities may not be able to access our laboratory or manufacturing space, and our core activities may be significantly limited or curtailed, possibly for an extended period of time.
As a result of the COVID-19 pandemic, or similar pandemics and outbreaks, we have and may in the future experience severe disruptions, including:
| • | interruption of or delays in receiving products and supplies from the third parties we rely on to, among other things, manufacture components of our products, due to staffing shortages, production slowdowns or stoppages and disruptions in delivery systems, which may impair our ability to manufacture and sell our products and provide our services; |
| • | limitations on our business operations by the local, state, or federal government that could impact our ability to manufacture, sell or deliver our products and services; |
| • | on-site visit limitations and prohibitions imposed by customers that could impact our ability to engage in pre-sales activities, and to provide post-sale activities, such as training and service and support; |
| • | delays in customers’ purchasing decisions and negotiations with customers and potential customers; |
| • | business disruptions caused by workplace, laboratory and office closures and an increased reliance on employees working from home, travel limitations, cyber security and data accessibility limits, or communication or mass transit disruptions; and |
| • | limitations on employee resources that would otherwise be focused on the conduct of our activities, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people. |
103
Any of these factors could severely impact our research and development activities, manufacturing business operations and sales, or delay necessary interactions with local regulators, third-party vendors and other important contractors and customers. These and other factors arising from the COVID-19 pandemic could worsen in countries that are already afflicted with COVID-19, could continue to spread to additional countries, or could return to countries where the pandemic has been partially contained, and could further adversely impact our ability to conduct our business generally and have a material adverse impact on our consolidated operations and financial condition and results. For example, our protein detection segment experienced a decline in sales during the second quarter of 2020 relative to the same period in 2019 due to stay-at-home orders in the San Francisco Bay Area and the closure of many academic laboratories that are the main customers of this segment and the reduced operations of other customers. Prolonged closures or shutdowns as a result of the COVID-19 pandemic would continue to affect sales of our protein detection segment adversely. For the nine months ended September 30, 2020, several of our product categories have experienced accelerated growth, notably our CleanCap® and oligonucleotide products. We expect the impact of COVID-19 on our growth to sustain in the longer term as the entire mRNA category benefits from lessons learned during the COVID-19 pandemic. We expect research in other therapeutic categories to experience increased growth as research conducted for COVID-19 diffuses more broadly into other vaccines and therapies.
The extent to which the pandemic may negatively impact our consolidated operations and results of operations or those of our third-party manufacturers, suppliers, partners or customers will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the duration of the pandemic, the extent of travel restrictions, additional or modified government actions, new information that will emerge concerning the severity and impact of COVID-19 and actions to contain the pandemic or treat its impact, such as social distancing, quarantines, lockdowns or business closures.
We have responded to the pandemic by leveraging our deep product portfolio and general scientific expertise to develop robust COVID-19-related product and service offerings providing critical support for the development of therapeutics, vaccines and diagnostics. Our ongoing efforts to utilize our portfolio of products and services to enable solutions for this evolving pandemic may offset the impact of our customer site closures.
We remain fully operational as we abide by local COVID-19 safety regulations across the world. To achieve this, we have many employees working remotely and have adopted significant protective measures for our employees on site, including staggered shifts, social distancing and hygiene best practices recommended by the Centers for Disease Control and Prevention (the “CDC”) and local public health officials. In addition, we have taken additional steps to monitor and strengthen our supply chain to maintain an uninterrupted supply of our critical products and services.
Organizational Transactions
Maravai LifeSciences Holdings, Inc. was incorporated in August 2020 and formed for the purpose of this offering and has engaged to date only in activities in contemplation of this offering. Maravai LifeSciences Holdings, Inc. will be a holding company and its sole material asset will be a controlling ownership interest in Topco LLC. For more information regarding our reorganization and holding company structure, see “Organizational Structure—Organizational Transactions.” Upon completion of this offering, all of our business will be conducted through Topco LLC and its consolidated subsidiaries, and the financial results of Topco LLC and its consolidated subsidiaries will be included in the consolidated financial statements of Maravai LifeSciences Holdings, Inc.
Topco LLC has been treated as a pass-through entity for U.S. federal and state income tax purposes and accordingly has not been subject to U.S. federal or state income tax. Certain wholly owned subsidiaries of Topco are taxed as corporations for U.S. federal and most applicable state, local income tax and foreign tax purposes. After consummation of this offering, Topco LLC will continue to be treated as a pass-through entity for U.S.
104
federal and state income tax purposes and certain subsidiaries will continue to be taxed as corporations for U.S. federal and most applicable state, local income tax and foreign tax purposes. As a result of its ownership of LLC Units in Topco LLC, Maravai LifeSciences Holdings, Inc. will become subject to U.S. federal, state and local income taxes with respect to its allocable share of any taxable income of Topco LLC and will be taxed at the prevailing corporate tax rates. In addition to tax expenses, we also will incur expenses related to our operations and we will be required to make payments under the Tax Receivable Agreement with MLSH 1 and MLSH 2. Due to the uncertainty of various factors, we cannot estimate the likely tax benefits we will realize as a result of LLC Unit exchanges, and the resulting amounts we are likely to pay out to LLC Unitholders pursuant to the Tax Receivable Agreement; however, we estimate that such payments may be substantial. We intend to cause Topco LLC to make distributions in an amount sufficient to allow us to pay our tax obligations and operating expenses, including distributions to fund any ordinary course payments due under the Tax Receivable Agreement. See ‘‘Organizational Structure—Amended and Restated Operating Agreement of Topco LLC’’ and ‘‘Organizational Structure—Tax Receivable Agreement.”
How We Assess Our Business
We consider a variety of financial and operating measures in assessing the performance of our business. The key measures we use to determine how our business is performing are revenue and Adjusted EBITDA.
Adjusted EBITDA is a non-GAAP financial measure that we define as net loss adjusted for interest expense, provision for income taxes, depreciation, amortization and unit-based compensation expenses. Adjusted EBITDA reflects further adjustments to eliminate the impact of certain items, including certain non-cash and other items, that we do not consider representative of our ongoing operating performance. We also present Adjusted Free Cash Flow, which is a non-GAAP measure that we define as Adjusted EBITDA less capital expenditures.
Management uses Adjusted EBITDA to evaluate the financial performance of our business and the effectiveness of our business strategies. We present Adjusted EBITDA and Adjusted Free Cash Flow because we believe they are frequently used by analysts, investors and other interested parties to evaluate companies in our industry and they facilitate comparisons on a consistent basis across reporting periods. Further, we believe they are helpful in highlighting trends in our operating results because they exclude items that are not indicative of our core operating performance. Adjusted EBITDA is also a component of the financial covenant under the New Credit Agreement that governs our ability to access more than $63.0 million in aggregate letters of credit obligations and outstanding borrowings under the New Revolving Credit Facility. In addition, if we borrow more than $63.0 million, we are required to maintain a specified net leverage ratio. See “—Liquidity and Capital Resources—Sources of Liquidity—Debt Covenants” for a discussion of this financial covenant.
Adjusted EBITDA and Adjusted Free Cash Flow have limitations as analytical tools and you should not consider them in isolation, or as substitutes for analysis of our results as reported under GAAP. We may in the future incur expenses similar to the adjustments in the presentation of Adjusted EBITDA. In particular, we expect to incur meaningful share-based compensation expense in the future. Other limitations include that Adjusted EBITDA and Adjusted Free Cash Flow do not reflect:
| • | all expenditures or future requirements for capital expenditures or contractual commitments; |
| • | changes in our working capital needs; |
| • | provision for income taxes, which may be a necessary element of our costs and ability to operate; |
| • | the costs of replacing the assets being depreciated, which will often have to be replaced in the future; |
| • | the non-cash component of employee compensation expense; and |
| • | the impact of earnings or charges resulting from matters we consider not to be reflective, on a recurring basis, of our ongoing operations. |
In addition, Adjusted EBITDA and Adjusted Free Cash Flow may not be comparable to similarly titled measures used by other companies in our industry or across different industries.
105
Components of Results of Operations
Revenue
Our revenue consists of both product and services revenue and, to a much lesser extent, revenue from royalties attributable to the out-licensing of our proprietary biological assets intellectual property that we may develop. We generated total consolidated revenue of $123.8 million and $143.1 million for the years ended December 31, 2018 and 2019, respectively, and $107.2 million and $185.7 million for the nine months ended September 30, 2019 and 2020, respectively, through the following segments: (i) nucleic acid production, (ii) biologics safety testing and (iii) protein detection.
Nucleic Acid Production Segment
Our nucleic acid production segment focuses on the manufacturing and sale of highly modified nucleic acids products to support the needs of customers’ research, therapeutic and vaccine programs. This segment also provides research products for labeling and detecting proteins in cells and tissue samples.
Biologics Safety Testing Segment
Our biologics safety testing segment focuses on manufacturing and selling biologics safety and impurity tests and assay development services that are utilized by our customers in their biologic drug manufacturing activities.
Protein Detection Segment
Our protein detection segment products, which include a portfolio of labeling and visual detection reagents, are purchased by our scientific research customers for their tissue-based protein detection and characterization needs.
Costs of Revenue
Cost of revenue associated with our products and services primarily consists of manufacturing related costs incurred in the production process, including personnel and related costs, unit-based compensation from awards issued by our parent and sole member, MLSH 1, and by one of our subsidiaries, inventory write-downs, costs of materials, labor and overhead, packaging and delivery costs and allocated costs, including facilities, information technology, depreciation, and amortization of intangibles. Cost of revenue associated with our services primarily consists of personnel and related costs, unit-based compensation from awards issued by our parent and sole member, cost of materials and allocated costs, including facilities and information technology costs. Costs of services were not material to the years ended December 31, 2018 and 2019 and the nine months ended September 30, 2019 and 2020.
We expect cost of revenue to increase in future periods as our revenue grows.
Operating Expenses
Research and development. Research and development costs primarily consist of salaries, benefits, incentive compensation, unit-based compensation from awards issued by MLSH 1 and one of our subsidiaries, cost of supplies, in-process research and development costs from asset acquisitions and allocated facilities costs for employees engaged in research and development of products and services. We expense all research and development costs in the period in which they are incurred. Payment made prior to the receipt of goods or services to be used in research and development are recognized as prepaid assets until the goods are received or services are rendered.
We plan to continue to support our research and development efforts, including meeting our customers’ customizable needs.
106
Selling, general and administrative. Our selling, general and administrative expenses primarily consist of salaries, benefits and unit-based compensation costs from awards issued by MLSH 1 and also one of our subsidiaries for employees in our commercial sales functions, marketing, executive, accounting and finance, legal and human resource functions as well as travel expenses, professional services fees, such as consulting, audit, tax and legal fees, general corporate costs and allocated costs, including facilities, information technology and amortization of intangibles.
We expect that our selling, general and administrative expenses will continue to increase after this offering, primarily due to increased headcount to support anticipated growth in the business, costs incurred in increasing our presence globally and increases in marketing activities to drive awareness and adoption of our products and services, and due to incremental costs associated with operating as a public company.
Other Income (Expense)
Other income (expense) consists primarily of interest related to borrowings under our long-term debt obligations. We also include foreign currency exchange gains and losses. During the year ended December 31, 2018, other income (expense) included the loss on extinguishment of debt, see Note 7 to our audited consolidated financial statements included elsewhere in this prospectus.
Noncontrolling Interest
Topco LLC’s consolidated financial statements as of December 31, 2018 and 2019 include a noncontrolling interest related to a minority interest in one of our subsidiaries. On September 16, 2020, we entered into various agreements with current investors in MLSC Holdings, LLC (“MLSC”), the parent of Cygnus Technologies, to repurchase 43,264 MLSC Class B preferred units and 18,387,206 MLSC common units from the investors for approximately $120.0 million. Additionally, the agreements provide that the remaining 16,736 MLSC Class B preferred units and 7,112,794 MLSC common units held by the investors party to the agreements are subject to exchange into a variable number of common units of MLSH 1 with a total fixed value of approximately $46.4 million. The repurchase and exchange shall take place immediately prior to, and is conditioned upon, the occurrence of this offering. If the repurchase of these Class B preferred units and common units is not consummated prior to January 16, 2021, the investors (other than the president of the non-controlling interest) have the right to request a reallocation (the “Reallocation Request Notice”) of their Class B preferred units and common units between those subject to repurchase and those subject to exchange. If the Reallocation Request Notice is not accepted and the repurchase does not occur within 14 days of receipt of the Reallocation Request Notes, such investors have the right to terminate the agreement.
In connection with the Organizational Transactions, Maravai LifeSciences Holdings, Inc. will be appointed as the sole managing member of Topco LLC pursuant to the amended and restated LLC Operating Agreement. Because we will manage and operate the business and control the strategic decisions and day-to-day operations of Topco LLC and will also have a substantial financial interest in Topco LLC, we will consolidate the financial results of Topco LLC, and a portion of our net income (loss) will be allocated to the noncontrolling interest to reflect the entitlement of the noncontrolling interest holders to Topco LLC’s net income (loss). We will hold approximately 32% of the outstanding LLC Units of Topco LLC (or approximately 34% of the outstanding LLC Units of Topco LLC if the underwriters exercise their option to purchase additional shares in full), and the outstanding LLC Units of Topco LLC will be held by MLSH 1.
Income Tax Expense (Benefit)
Topco LLC is currently, and will through consummation of the Organizational Transactions, be treated as a partnership for U.S. federal and most applicable state and local income tax purposes. As a partnership, its taxable income or loss is passed through to and included in the tax returns of its members, including us. Certain wholly
107
owned subsidiaries of Topco LLC are organized and treated as corporations for U.S. federal and most applicable state, local income tax and foreign tax purposes. Accordingly, the consolidated financial statements of Topco LLC included in this prospectus include a tax provision for federal, state, local and foreign income taxes.
For a description of the Tax Receivable Agreement, see “Organizational Structure—Tax Receivable Agreement.”
Future Public Company Expenses
We expect our operating expenses to increase when we become a public company following this offering. We expect our accounting, legal and personnel-related expenses and directors’ and officers’ insurance costs reported within selling, general and administrative to increase as we establish more comprehensive compliance and governance functions, maintain and review internal controls over financial reporting in accordance with the Sarbanes-Oxley Act and prepare and distribute periodic reports as required by the rules and regulations of the SEC. As a result, our historical results of operations may not be indicative of our results of operations in future periods.
Results of Operations for the Nine Months Ended September 30, 2019 and 2020
The results of operations presented below should be reviewed in conjunction with the condensed consolidated financial statements and notes included elsewhere in the prospectus. The following tables set forth our results of operations for the periods presented:
| Nine months ended September 30, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands except unit and per unit amounts |
2019 | 2020 | $ | % | 2019 | 2020 | ||||||||||||||||||
| Revenues |
$ | 107,180 | $ | 185,745 | $ | 78,565 | 73.3 | % | 100.0 | % | 100.0 | % | ||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Cost of revenue(1) |
49,019 | 56,254 | 7,235 | 14.8 | % | 45.7 | % | 30.3 | % | |||||||||||||||
| Research and development(1) |
2,648 | 7,212 | 4,564 | 172.4 | % | 2.5 | % | 3.9 | % | |||||||||||||||
| Selling, general and administrative(1) |
32,570 | 52,624 | 20,054 | 61.6 | % | 30.4 | % | 28.3 | % | |||||||||||||||
| Change in estimated fair value of contingent consideration |
241 | — | (241 | ) | * | * | * | |||||||||||||||||
| Gain on sale and leaseback transaction |
— | (19,002 | ) | (19,002 | ) | * | * | 10.2 | % | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Total operating costs and expenses |
84,478 | 97,088 | 12,610 | 14.9 | % | 78.8 | % | 52.3 | % | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Income from operations |
22,702 | 88,657 | 65,955 | 290.5 | % | 21.2 | % | 47.7 | % | |||||||||||||||
| Other income (expense) |
(22,145 | ) | (21,802 | ) | 343 | (1.5 | %) | (20.7 | %) | (11.7 | %) | |||||||||||||
| Income before income taxes |
557 | 66,855 | 66,298 | * | 0.5 | % | 36.0 | % | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Income tax expense |
308 | 2,511 | 2,203 | * | * | 1.4 | % | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net income |
249 | 64,344 | 64,095 | * | 0.2 | % | 34.6 | % | ||||||||||||||||
| Net (loss) income attributable to noncontrolling interests |
(672 | ) | 582 | 1,254 | * | |||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net income attributable to the Maravai Topco Holdings, LLC member |
$ | 921 | $ | 63,762 | $ | 62,841 | * | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net (loss) income per common unit attributable to Maravai Topco Holdings, LLC member—basic and diluted |
$ | (0.01 | ) | $ | 0.21 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Weighted average common units outstanding |
253,916,941 | 253,916,941 | ||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Non-GAAP measure: |
||||||||||||||||||||||||
| Adjusted EBITDA |
$ | 48,150 | $ | 104,840 | 56,690 | 117.7 | % | 44.9 | % | 56.4 | % | |||||||||||||
| Adjusted Free Cash Flow |
$ | 34,713 | $ | 83,118 | 48,405 | 139.4 | % | 32.4 | % | 44.7 | % | |||||||||||||
| * | Not meaningful |
108
| (1) | Amounts include unit-based compensation as follows: |
| Nine months ended September 30, |
Change | |||||||||||||||
| 2019 | 2020 | $ | % | |||||||||||||
| Cost of revenue |
16 | 11 | (5 | ) | (31.3 | )% | ||||||||||
| Research and development |
157 | 732 | 575 | 366.3 | % | |||||||||||
| Selling, general and administrative |
990 | 2,190 | 1,200 | 121.2 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total unit-based compensation |
$ | 1,163 | $ | 2,933 | $ | 1,770 | 152.2 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Revenue
Consolidated revenue by segment was as follows:
| Nine months ended September 30, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands |
2019 | 2020 | $ | % | 2019 | 2020 | ||||||||||||||||||
| Revenues |
||||||||||||||||||||||||
| Nucleic acid production |
$ | 54,867 | $ | 128,569 | $ | 73,702 | 134.3 | % | 51.2 | % | 69.2 | % | ||||||||||||
| Biologics safety testing |
32,819 | 40,772 | 7,953 | 24.2 | % | 30.6 | % | 22.0 | % | |||||||||||||||
| Protein detection |
19,494 | 16,404 | (3,090 | ) | (15.9 | %) | 18.2 | % | 8.8 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenue |
$ | 107,180 | $ | 185,745 | $ | 78,565 | 73.3 | % | 100.0 | % | 100.0 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Total revenue was $107.2 million for the nine months ended September 30, 2019 compared to $185.7 million for the nine months ended September 30, 2020, representing an increase of $78.6 million, or 73.3%.
Nucleic acid production revenue increased from $54.9 million for the nine months ended September 30, 2019 to $128.6 million for the nine months ended September 30, 2020, representing an increase of $73.7 million, or 134.3%. The increase in nucleic acid production was driven by increased demand for our proprietary CleanCap® analogs, which principally serve the growing mRNA vaccine and therapeutic markets and demand for our oligonucleotide synthesis inputs.
Biologics safety testing revenue increased from $32.8 million for the nine months ended September 30, 2019 to $40.8 million for the nine months ended September 30, 2020, representing an increase of $8.0 million, or 24.2%. The increase was driven by a continued increase in the number of bioproduction programs and customers that use our catalog of HCP ELISA kits.
Protein detection revenue decreased from $19.5 million for the nine months ended September 30, 2019 to $16.4 million for the nine months ended September 30, 2020, representing a decrease of $3.1 million, or 15.9%. The decrease was driven by lower sales for mounting media, antibodies, and avidin-biotin complex kits, partially offset by our bioconjugation product that launched in 2020.
Adjusted EBITDA and Segment Information
Management has determined that adjusted earnings before interest, tax, depreciation, and amortization is the profit or loss measure used to make resource allocation decisions and evaluate segment performance. Adjusted EBITDA assists management in comparing the segment performance on a consistent basis for purposes of business decision-making by removing the impact of certain items that management believes do not directly reflect the core operations and, therefore, are not included in measuring segment performance. Corporate costs are managed on a standalone basis and not allocated to segments.
109
Following is financial information relating to the operating segments (in thousands):
| As of and for the nine months ended |
Nucleica Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Eliminations | Total | ||||||||||||||||||
| Revenue |
$ | 54,867 | $ | 32,819 | $ | 19,494 | $ | — | $ | — | $ | 107,180 | ||||||||||||
| Adjusted EBITDA |
$ | 18,551 | $ | 26,961 | $ | 10,841 | $ | (8,203 | ) | $ | — | $ | 48,150 | |||||||||||
| As of and for the nine months ended |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Eliminations | Total | ||||||||||||||||||
| Revenue |
$ | 129,645 | $ | 40,772 | $ | 16,404 | $ | — | $ | (1,076 | ) | $ | 185,745 | |||||||||||
| Adjusted EBITDA |
$ | 76,130 | $ | 33,571 | $ | 6,960 | $ | (11,597 | ) | $ | (224 | ) | $ | 104,840 | ||||||||||
There was no inter-segment activity for the nine months ended September 30, 2019. During the nine-month period ended September 30, 2020, intersegment revenue was $1.1 million. The intersegment sales and the related gross margin on inventory recorded at the end of the period are eliminated for consolidation purposes in the Eliminations column. Internal selling prices for intersegment sales are consistent with the segment’s normal retail price offered to external parties. There was no commission expense recognized for intersegment sales for the nine months ended September 30, 2020. Intersegment revenue represents intersegment revenue between the Nucleic Acid Production and Protein Detection segments.
We do not allocate assets to our reportable segments as they are not included in the review performed by our Chief Operating Decision Maker for purposes of assessing segment performance and allocating resources.
Excluding approximately $0.3 million associated with a building in the United Kingdom, all of our long-livedassets are located within the United States.
A reconciliation of Adjusted EBITDA to net income, the most directly comparable GAAP measure, is set forth below (in thousands):
| For the nine months ended September 30, |
||||||||
| 2019 | 2020 | |||||||
| Net Income |
$ | 249 | $ | 64,344 | ||||
| Add: |
||||||||
| Amortization |
15,118 | 15,156 | ||||||
| Depreciation |
2,331 | 4,756 | ||||||
| Interest expense |
22,240 | 21,934 | ||||||
| Income tax expense |
308 | 2,511 | ||||||
|
|
|
|
|
|||||
| EBITDA |
40,246 | 108,701 | ||||||
| Acquisition contingent consideration(a) |
241 | — | ||||||
| Acquisition integration costs(b) |
4,061 | 3,588 | ||||||
| Amortization of purchase accounting inventory step-up(c) |
1,856 | — | ||||||
| Acquired in-process research and development costs(d) |
— | 2,881 | ||||||
| Unit-based compensation(e) |
1,163 | 2,933 | ||||||
| GTCR management fees(f) |
421 | 555 | ||||||
| Gain on sale and leaseback transaction(g) |
— | (19,002 | ) | |||||
| Merger and acquisition related expenses(h) |
162 | 218 | ||||||
| Financing costs(i) |
— | 4,966 | ||||||
|
|
|
|
|
|||||
| Adjusted EBITDA |
$ | 48,150 | $ | 104,840 | ||||
|
|
|
|
|
|||||
| (a) | Refers to the change in fair value and settlement of earnout payments related to a 2017 acquisition. |
| (b) | Refers to incremental costs incurred to execute and integrate completed acquisitions. |
110
| (c) | Refers to a non-cash charge related to the amortization expense of the step-up of inventory from purchase price accounting. |
| (d) | Refers to in-process research and development charge associated with the acquisition of MockV Solutions, Inc. |
| (e) | Refers to non-cash expense associated with unit-based compensation. |
| (f) | Refers to cash fees paid to GTCR, pursuant to the advisory services agreement that will terminate in connection with this offering. |
| (g) | Refers to the gain on the sale of our Burlingame, California facility, which was leased back to the Company in 2020. |
| (h) | Refers to diligence, legal, accounting, tax and consulting fees incurred associated with acquisitions that were not consummated. |
| (i) | Refers to transaction costs related to this offering and the refinancing of our Credit Facilities that are not capitalizable or cannot be offset against proceeds from such transactions. |
Adjusted Free Cash Flow
Adjusted Free Cash Flow, which is a non-GAAP measure that we define as Adjusted EBITDA less capital expenditures, is set forth below (in thousands):
| For the nine months ended September 30, |
||||||||
| 2019 | 2020 | |||||||
| Adjusted EBITDA |
$ | 48,150 | $ | 104,840 | ||||
| Capital expenditures(a) |
(13,437 | ) | $ | (21,722 | ) | |||
|
|
|
|
|
|||||
| Adjusted Free Cash Flow |
$ | 34,713 | $ | 83,118 | ||||
|
|
|
|
|
|||||
| (a) | We define capital expenditures as purchases of property and equipment, which are included in cash flows from investing activities, and accounts payable and accrued expenses. |
Costs of Revenue
| Nine months ended September 30, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands | 2019 | 2020 | $ | % | 2019 | 2020 | ||||||||||||||||||
| Cost of revenue |
$ | 49,019 | $ | 56,254 | $ | 7,235 | 14.8 | % | 45.7 | % | 30.3 | % | ||||||||||||
Cost of revenue increased by $7.2 million from $49.0 million for the nine months ended September 30, 2019 to $56.2 million for the nine months ended September 30, 2020, or 14.8%. The increase in cost of revenue was primarily attributable to increases in direct product costs based on higher revenue.
Research and Development
| Nine months ended September 30, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands | 2019 | 2020 | $ | % | 2019 | 2020 | ||||||||||||||||||
| Research and development |
$ | 2,648 | $ | 7,212 | $ | 4,564 | 172.4 | % | 2.5 | % | 3.9 | % | ||||||||||||
Research and development expenses increased by $4.6 million from $2.6 million for the nine months ended September 30, 2019 compared to $7.2 million for the nine months ended September 30, 2020, or 172.3%. The increase was primarily attributable to a $2.9 million increase in in-process research and development costs related to an asset acquisition in addition to a $0.9 million increase in personnel costs primarily due to the increases in unit-based compensation expense related to the agreement to repurchase MLSC incentive units.
111
Selling, General and Administrative
| Nine months ended September 30, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands | 2019 | 2020 | $ | % | 2019 | 2020 | ||||||||||||||||||
| Selling, general and administrative |
$ | 32,570 | $ | 52,624 | $ | 20,054 | 61.6 | % | 30.4 | % | 28.3 | % | ||||||||||||
Selling, general and administrative expenses were $32.6 million for the nine months ended September 30, 2019 compared to $52.6 million for the nine months ended September 30, 2020, representing an increase of $20.0 million, or 61.6%. The increase was primarily due to a $7.8 million increase in personnel costs, as we increased our headcount, a $2.0 million increase in facility and information technology costs to support our increased headcount, and an $8.4 million increase in professional services costs. Professional services in the nine months ended September 30, 2020 include nonrecurring costs on acquisition activity and costs associated with preparing to operating as a public company.
Other Income (Expense)
| Nine months ended September 30, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands | 2019 | 2020 | $ | % | 2019 | 2020 | ||||||||||||||||||
| Other income (expense) |
||||||||||||||||||||||||
| Interest expense |
$ | (22,240 | ) | $ | (21,934 | ) | $ | 306 | (1.4 | %) | (20.8 | %) | (11.8 | %) | ||||||||||
| Other income |
95 | 132 | 37 | 38.9 | % | 0.1 | % | 0.1 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total other income (expense) |
$ | (22,145 | ) | $ | (21,802 | ) | $ | 343 | (1.5 | %) | (20.7 | %) | (11.7 | %) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Other expense was $22.1 million for the nine months ended September 30, 2019 compared to $21.8 million for the nine months ended September 30, 2020, representing a decrease of $0.3 million, or (1.5%). The decrease in expense was primarily attributable to declining variable interest rates on outstanding debt.
112
Results of Operations for the Years Ended December 31, 2018 and 2019
The results of operations presented below should be reviewed in conjunction with the consolidated financial statements and notes included elsewhere in the prospectus. The following tables set forth our results of operations for the periods presented:
| Year ended December 31, | Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands except unit and per unit amounts |
2018 | 2019 | $ | % | 2018 | 2019 | ||||||||||||||||||
| Revenue |
$ | 123,833 | $ | 143,140 | $ | 19,307 | 15.6 | % | 100.0 | % | 100.0 | % | ||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Cost of revenue(1) |
60,765 | 66,849 | 6,084 | 10.0 | % | 49.1 | % | 46.7 | % | |||||||||||||||
| Research and development(1) |
4,499 | 3,627 | (872 | ) | (19.4 | %) | 3.6 | % | 2.5 | % | ||||||||||||||
| Selling, general and administrative(1) |
41,194 | 48,354 | 7,160 | 17.4 | % | 33.3 | % | 33.8 | % | |||||||||||||||
| Change in estimated fair value of contingent consideration |
939 | 322 | (617 | ) | (65.7 | %) | 0.8 | % | 0.2 | % | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Total operating costs and expenses |
107,397 | 119,152 | 11,755 | 10.9 | % | 86.7 | % | 83.2 | % | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Income from operations |
16,436 | 23,988 | 7,552 | 45.9 | % | 13.3 | % | 16.8 | % | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Other income (expense) |
(32,934 | ) | (29,841 | ) | 3,093 | (9.4 | %) | (26.6 | %) | (20.8 | %) | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Loss before income taxes |
(16,498 | ) | (5,853 | ) | 10,645 | (64.5 | %) | (13.3 | %) | (4.1 | %) | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Income tax expense (benefit) |
417 | (652 | ) | (1,069 | ) | (256.4 | %) | 0.3 | % | (0.5 | %) | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net loss |
$ | (16,915 | ) | $ | (5,201 | ) | $ | 11,714 | (69.3 | %) | (13.7 | %) | (3.6 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net loss attributable to noncontrolling interests |
(12,443 | ) | (731 | ) | 11,712 | (94.1 | %) | |||||||||||||||||
| Net loss attributable to the Maravai Topco Holdings, LLC member |
(4,472 | ) | (4,470 | ) | 2 | 0.0 | % | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net loss per common unit attributable to Maravai Topco Holdings, LLC member—basic and diluted |
$ | (0.07 | ) | $ | (0.03 | ) | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Weighted average common units outstanding |
253,916,941 | 253,916,941 | ||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Non-GAAP measure: |
||||||||||||||||||||||||
| Adjusted EBITDA |
$ | 53,000 | $ | 62,014 | 9,014 | 17.0 | % | 42.8 | % | 43.3 | % | |||||||||||||
| Adjusted Free Cash Flow |
$ | 49,263 | $ | 42,101 | $ | (7,092 | ) | (14.4 | )% | 39.7 | % | 29.4 | % | |||||||||||
| (1) | Amounts include unit-based compensation as follows: |
| Year ended December 31, |
Change | |||||||||||||||
| in thousands |
2018 | 2019 | $ | % | ||||||||||||
| Cost of revenue |
$ | 38 | $ | 22 | (16 | ) | (42.1 | %) | ||||||||
| Research and development |
297 | 211 | (86 | ) | (29.0 | %) | ||||||||||
| Selling, general and administrative |
1,786 | 1,446 | (340 | ) | (19.0 | %) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total unit-based compensation expense |
$ | 2,121 | $ | 1,679 | $ | (442 | ) | (20.8 | %) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
113
Revenue
Consolidated revenue by segment were as follows:
| Year ended December 31, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands |
2018 | 2019 | $ | % | 2018 | 2019 | ||||||||||||||||||
| Revenue |
||||||||||||||||||||||||
| Nucleic acid production |
$ | 60,057 | $ | 72,602 | $ | 12,545 | 20.9 | % | 48.5 | % | 50.7 | % | ||||||||||||
| Biologics safety testing |
38,492 | 44,416 | 5,924 | 15.4 | % | 31.1 | % | 31.0 | % | |||||||||||||||
| Protein detection |
25,284 | 26,122 | 838 | 3.3 | % | 20.4 | % | 18.3 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenue |
$ | 123,833 | $ | 143,140 | $ | 19,307 | 15.6 | % | 100.0 | % | 100.0 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Total revenue was $123.8 million for the year ended December 31, 2018 compared to $143.1 million for the year ended December 31, 2019, representing an increase of $19.3 million, or 15.6%.
Nucleic acid production revenue increased from $60.1 million for the year ended December 31, 2018 to $72.6 million for the year ended December 31, 2019, representing an increase of $12.5 million, or 20.9%. The increase in nucleic acid production was driven by increased demand for highly modified RNA products, particularly mRNA, as well as increased demand for our proprietary CleanCap® analogs, which principally serve the growing mRNA vaccine and therapeutic markets.
Biologics safety testing revenue increased from $38.5 million for the year ended December 31, 2018 to $44.4 million for the year ended December 31, 2019, representing an increase of $5.9 million, or 15.4%. The increase was driven by a continued increase in the number of bioproduction programs and customers that use our catalog of HCP ELISA kits.
Protein detection revenue increased from $25.3 million for the year ended December 31, 2018 to $26.1 million for the year ended December 31, 2019, representing an increase of $0.8 million, or 3.3%. The increase was driven by strong sales for lectins and glycobiology reagents, histology reagents and blocking reagents.
Adjusted EBITDA and Segment Information
Management has determined that adjusted earnings before interest, tax, depreciation, and amortization is the profit or loss measure used to make resource allocation decisions and evaluate segment performance. Adjusted EBITDA assists management in comparing the segment performance on a consistent basis for purposes of business decision-making by removing the impact of certain items that management believes do not directly reflect the core operations and, therefore, are not included in measuring segment performance. Corporate costs are managed on a standalone basis and not allocated to segments.
Following is financial information relating to the operating segments (in thousands):
| As of and for the year ended December 31, 2018 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Total | |||||||||||||||
| Revenue |
$ | 60,057 | $ | 38,492 | $ | 25,284 | $ | — | $ | 123,833 | ||||||||||
| Adjusted EBITDA |
$ | 16,751 | $ | 31,199 | $ | 13,846 | $ | (8,796 | ) | $ | 53,000 | |||||||||
| As of and for the year ended December 31, 2019 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Total | |||||||||||||||
| Revenue |
$ | 72,602 | $ | 44,416 | $ | 26,122 | $ | — | $ | 143,140 | ||||||||||
| Adjusted EBITDA |
$ | 22,229 | $ | 36,371 | $ | 14,603 | $ | (11,189 | ) | $ | 62,014 | |||||||||
114
There was no inter-segment activity for any of the periods presented and all of the revenue for each segment is from external customers.
We do not allocate assets to our reportable segments as they are not included in the review performed by our Chief Operating Decision Maker for purposes of assessing segment performance and allocating resources. Excluding approximately $0.3 million associated with a building in the United Kingdom, all of our long-lived assets are located within the United States.
A reconciliation of Adjusted EBITDA to net loss, the most directly comparable GAAP measure, is set forth below (in thousands):
| Year Ended December 31, |
||||||||
| 2018 | 2019 | |||||||
| Net Loss |
$ | (16,915 | ) | $ | (5,201 | ) | ||
| Add: |
||||||||
| Amortization |
20,122 | 20,274 | ||||||
| Depreciation |
2,225 | 3,810 | ||||||
| Interest expense |
27,399 | 29,959 | ||||||
| Income tax (benefit) expense |
417 | (652 | ) | |||||
|
|
|
|
|
|||||
| EBITDA |
33,248 | 48,190 | ||||||
| Acquisition contingent consideration(a) |
939 | 322 | ||||||
| Loss on extinguishment of debt(b) |
5,622 | — | ||||||
| Acquisition integration costs(c) |
7,529 | 6,170 | ||||||
| Amortization of purchase accounting inventory step-up(d) |
2,967 | 1,856 | ||||||
| Unit-based compensation(e) |
2,121 | 1,679 | ||||||
| GTCR management fees(f) |
574 | 523 | ||||||
| Merger and acquisition related expenses(g) |
— | 3,274 | ||||||
|
|
|
|
|
|||||
| Adjusted EBITDA |
$ | 53,000 | $ | 62,014 | ||||
|
|
|
|
|
|||||
| (a) | Refers to the change in fair value and settlement of earnout payments related to a 2017 acquisition. |
| (b) | Refers to non-operating cash expense incurred on extinguishment of debt. |
| (c) | Refers to incremental costs incurred to execute and integrate completed acquisitions. |
| (d) | Refers to a non-cash charge related to the amortization expense of the step-up of inventory from purchase price accounting. |
| (e) | Refers to non-cash expense associated with unit-based compensation. |
| (f) | Refers to cash fees paid to GTCR, pursuant to the advisory services agreement that will terminate in connection with this offering. |
| (g) | Refers to diligence, legal, accounting, tax and consulting fees incurred associated with an acquisition that was not consummated. |
115
Adjusted Free Cash Flow
Adjusted Free Cash Flow, which is a non-GAAP measure that we define as Adjusted EBITDA less capital expenditures, is set forth below (in thousands):
| Year Ended December 31, |
||||||||
| 2018 | 2019 | |||||||
| Adjusted EBITDA |
$ | 53,000 | $ | 62,014 | ||||
| Capital expenditures(a) |
$ | (3,737 | ) | $ | (19,913 | ) | ||
|
|
|
|
|
|||||
| Adjusted Free Cash Flow |
$ | 49,263 | $ | 42,101 | ||||
|
|
|
|
|
|||||
| (a) | We define capital expenditures as purchases of property and equipment, which are included in cash flows from investing activities, and accounts payable and accrued expenses. |
Costs of Revenue
| Year ended December 31, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands |
2018 | 2019 | $ | % | 2018 | 2019 | ||||||||||||||||||
| Cost of revenue |
$ | 60,765 | $ | 66,849 | $ | 6,084 | 10.0 | % | 49.1 | % | 46.7 | % | ||||||||||||
Cost of revenue increased by $6.0 million from $60.8 million for the year ended December 31, 2018 to $66.8 million for the year ended December 31, 2019, or 10.0%. The increase in cost of revenue was primarily attributable to increases in direct product costs, personnel costs, and supplies and materials costs, as margins were generally consistent.
Research and Development
| Year ended December 31, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands |
2018 | 2019 | $ | % | 2018 | 2019 | ||||||||||||||||||
| Research and development |
$ | 4,499 | $ | 3,627 | $ | (872 | ) | (19.4 | %) | 3.6 | % | 2.5 | % | |||||||||||
Research and development expenses decreased by $0.9 million from $4.5 million for the year ended December 31, 2018 compared to $3.6 million for the year ended December 31, 2019, or 19.4%. The decrease was primarily attributable to a $0.6 million decrease in personnel costs due to a reduction in headcount and a $0.3 million decrease in facilities and information technology allocation.
Selling, General and Administrative
| Year ended December 31, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands |
2018 | 2019 | $ | % | 2018 | 2019 | ||||||||||||||||||
| Selling, general and administrative |
$ | 41,194 | $ | 48,354 | $ | 7,160 | 17.4 | % | 33.3 | % | 33.8 | % | ||||||||||||
Selling, general and administrative expenses were $41.2 million for the year ended December 31, 2018 compared to $48.4 million for the year ended December 31, 2019, representing an increase of $7.2 million, or 17.4%. The increase was due to a $2.5 million increase in personnel costs as we increased our headcount, a $0.9 million increase in facility and information technology costs to support our increased headcount, a
116
$2.4 million increase in reimbursable expenses passed through from GTCR associated with certain business development activities (See “Related Party Transactions”) and an increase in professional services costs of $0.6 million.
Other Income (Expense)
| Year ended December 31, |
Change | Percentage of Revenue |
||||||||||||||||||||||
| in thousands |
2018 | 2019 | $ | % | 2018 | 2019 | ||||||||||||||||||
| Other income (expense) |
||||||||||||||||||||||||
| Interest expense |
$ | (27,399 | ) | $ | (29,959 | ) | $ | (2,560 | ) | 9.3 | % | (22.1 | %) | (20.9 | %) | |||||||||
| Loss on extinguishment of debt |
(5,622 | ) | — | 5,622 | (100.0 | %) | (4.5 | %) | 0.0 | % | ||||||||||||||
| Other income |
87 | 118 | 31 | 35.6 | % | 0.1 | % | 0.1 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total other income (expense) |
$ | (32,934 | ) | $ | (29,841 | ) | $ | 3,093 | (9.4 | %) | (26.6 | %) | (20.8 | %) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Other expense was $32.9 million for the year ended December 31, 2018 compared to $29.8 million for the year ended December 31, 2019, representing a decrease of $3.1 million, or 9.4%. The decrease was primarily attributable due to a $2.6 million increase in interest expense offset by a non-recurring loss on extinguishment of debt of $5.6 million recorded for the year ended December 31, 2018 in connection with the entry into the First Lien Credit Agreement and Second Lien Credit Agreement to refinance existing indebtedness.
Relationship with Our Sponsor
We have utilized GTCR, who upon completion of this offering will control the vote of all matters submitted to a vote of our shareholders, for certain services pursuant to an advisory services agreement. Under this agreement, GTCR provides us with financial and management consulting services in the areas of corporate strategy, budgeting for future corporate investments, acquisition and divestiture strategies and debt and equity financings. The advisory services agreement provides that we pay a $0.1 million quarterly management fee to GTCR for these services. We also reimburse GTCR for out-of-pocket expenses incurred while providing these services. The advisory services agreement also provides that certain of our subsidiaries pay placement fees to GTCR of 1.0% of the gross amount of debt or equity financings. The advisory services agreement will terminate in connection with this offering.
We paid GTCR $0.6 million in each of the years ended December 31, 2018 and 2019 and $0.4 million and $0.5 million during the nine months ended September 30, 2019 and 2020, respectively, for services in connection with the advisory services agreement. In October 2020, we paid GTCR a placement fee of $3.7 million in connection with entering into the New Credit Agreement. Following this offering, we may continue to engage GTCR from time to time, subject to compliance with our related party transactions policy.
During the year ended December 31, 2018, $52.0 million of capital distributions were made to the Class A unit holders of MLSC, including GTCR. No distributions were made to the Class A unit holders of MLSC Holdings, LLC during the year ended December 31, 2019. During the nine months ended September 30, 2020, $0.3 million of tax distributions were made to certain holders of MLSC incentive units. During the nine months ended September 30, 2020, no distributions were made to Class A or Class B preferred unitholders of MLSC or to MLSH 1.
In October 2020, we paid an $88.6 million distribution to MLSH 1. The distribution was fully funded through the use of cash on hand as of September 30, 2020 and cash flows generated from the New Credit Agreement. See “Liquidity and Capital Resources.”
117
Liquidity and Capital Resources
Overview
We have experienced losses in each full fiscal year since our inception. However, we had positive cash flows from operations for the year ended December 31, 2019. For the year ended December 31, 2019, we had a consolidated net loss of $5.2 million and had an accumulated deficit of $42.4 million as of December 31, 2019. We had positive cash flows from operations for the nine months ended September 30, 2019 and 2020 of $14.9 million and $72.5 million, respectively. For the nine months ended September 30, 2020, we had consolidated net income of $64.3 million.
We have relied on revenue derived from product and services sales and equity and debt financings to fund our operations to date, including the $310.6 million refinancing of our Credit Facilities (see Note 7 to the audited consolidated financial statements included elsewhere in this prospectus) in 2018 which was used to repay our legacy credit facility, senior subordinated notes and term loan and make a $52.0 million distribution to our member. In addition, on October 19, 2020, we refinanced our existing $400.0 million debt facilities with a new $780.0 million facility, which provided for the full repayment of $363.0 million of pre-existing debt and accrued interest under the Credit Facilities, the payment of the repurchase liability for incentive units and the repurchase liability for noncontrolling interests to be settled in cash $9.1 million and $120.0 million, respectively (see Notes 8 and 9 to the unaudited condensed consolidated financial statements included elsewhere in this prospectus) and to allow for an $88.6 million distribution to our member for various incentive unit holders of MLSH 1, which represents a return of capital which will result in a reduction of future amounts returned to such unit holders upon the closing of this offering. As of September 30, 2020, we had cash of $124.9 million and retained earnings of $21.4 million, as compared to cash of $24.7 million and $42.4 million of accumulated deficit as of December 31, 2019 and cash of $21.9 million and an accumulated deficit of $38.2 million as of December 31, 2018.
Our principal uses of cash have been to fund operations, acquisitions and capital expenditures, as well as make distributions to our member, interest payments and mandatory principal payments on our long-term debt.
Based on our current business plan, we believe the net proceeds from this offering, together with our existing cash and anticipated cash flows from operations will be sufficient to meet our working capital and capital expenditure needs over at least the next 12 months following the date of this prospectus.
We plan to utilize our existing cash on hand, together with cash generated from operations, primarily to fund our commercial and marketing activities associated with our products and services, continued research and development initiatives, and ongoing investments into our manufacturing facilities to create efficiencies and build capacity.
To the extent revenue from sales in our three business segments continue to grow, we expect our accounts receivable and inventory balances to increase. Any increase in accounts receivable and inventory may not be completely offset by increases in accounts payable and accrued expenses, which could result in greater working capital requirements. Moreover, following the closing of this offering, we expect to incur additional costs associated with operating as a public company, including expenses related to legal, accounting, regulatory, exchange listing and SEC compliance matters.
Our future capital requirements will depend on many factors including, but not limited to, our ability to successfully develop and launch new products and services, and to achieve a level of sales adequate to support our cost structure. If we are unable to execute on our business plan and adequately fund operations, or if the business plan requires a level of spending in excess of cash resources, we may seek additional equity, equity-linked or debt financing. If additional financings are required from outside sources, we may not be able to raise additional capital on terms acceptable to us or at all. If we are unable to raise additional capital when desired, our business, financial condition, results of operations and prospects could be adversely affected.
118
As a result of its ownership of LLC Units in Topco LLC, Maravai LifeSciences Holdings, Inc. will become subject to U.S. federal, state and local income taxes with respect to its allocable share of any taxable income of Topco LLC and will be taxed at the prevailing corporate tax rates. In addition to tax expenses, we also will incur expenses related to our operations and we will be required to make payments under the Tax Receivable Agreement with MLSH 1 and MLSH 2. Due to the uncertainty of various factors, we cannot precisely quantify the likely tax benefits we will realize as a result of LLC Unit exchanges and the resulting amounts we are likely to pay out to LLC Unitholders pursuant to the Tax Receivable Agreement; however, we estimate that such payments may be substantial. Assuming no changes in the relevant tax law, and that we earn sufficient taxable income to realize all tax benefits that are subject to the Tax Receivable Agreement, we expect that future payments under the Tax Receivable Agreement relating to the purchase by Maravai LifeSciences Holdings, Inc. of LLC Units from MLSH 1 in connection with this offering to be approximately $279.8 million (or approximately $317.8 million if the underwriters exercise their option to purchase additional shares, the proceeds of which will be used by Maravai LifeSciences Holdings, Inc. to acquire additional LLC Units from MLSH 1 and shares of Class A common stock from MLSH 2) and to range over the next 15 years from approximately $5.3 million to $21.2 million per year (or range from approximately $6.0 million to $24.1 million per year if the underwriters exercise their option to purchase additional shares) and decline thereafter. As a result, we expect that aggregate payments under the Tax Receivable Agreement over this 15-year period will be approximately $279.8 million (or approximately $317.8 million if the underwriters exercise their option to purchase additional shares). These estimates are based on an initial public offering price of $25.50 per share of Class A common stock, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus. Future payments in respect of subsequent exchanges or financings would be in addition to these amounts and are expected to be substantial. The foregoing numbers are merely estimates and the actual payments could differ materially. We expect to fund these payments using cash on hand and cash generated from operations. See “Organizational Structure—Amended and Restated Operating Agreement of Topco LLC” and “Organizational Structure—Tax Receivable Agreement.”
Sources of Liquidity
Since our inception, we have financed our operations primarily from the issuance of capital units, borrowings under long-term debt agreements and, to a lesser extent, cash flow from operations.
First and Second Lien Credit Agreement
In August of 2018, Maravai Intermediate Holdings, LLC (“Intermediate”), a wholly-owned subsidiary of ours, along with its subsidiaries Vector Laboratories, TriLink Biotechnologies and Cygnus Technologies (together with Intermediate, the “Borrowers”) entered into a first lien credit agreement (the “First Lien Credit Agreement”) with leading institutions for term loan borrowings (the “First Lien Term Loan”) totaling $250.0 million and a second lien credit agreement (the “Second Lien Credit Agreement”) for term loan borrowings (the “Second Lien Term Loan”) totaling $100.0 million, to refinance a combined debt agreement entered into in 2017, including repayment of all outstanding senior secured credit facilities and senior subordinated notes outstanding and to allow for a $52.0 million distribution to our members. The First Lien Credit Agreement also provided for a revolving credit facility (the “Revolving Credit Facility”) of $50.0 million for letters of credit and loans to be used for working capital and other general corporate financing purposes. Borrowings under the First Lien Credit Agreement and the Second Lien Credit Agreement were unconditionally guaranteed by Topco LLC and the existing and future material domestic subsidiaries of Topco LLC (subject to certain exceptions as specified in the respective guaranty agreements, and are secured by a lien and security interest in substantially all of the assets of existing and future material domestic subsidiaries of Topco LLC that are loan parties.
The First Lien Term Loan became repayable in quarterly payments of $0.6 million beginning December 31, 2018. Borrowings under the First Lien Credit Agreement bore interest (a) in the case of the First Lien Term Loans, at the Borrowers’ option, either at (i) the Base Rate plus the applicable margin of 3.25% per annum or
119
(ii) the Adjusted Eurocurrency Rate plus the margin of 4.25% per annum and (b) in the case of the Revolving Credit Facility, at the Borrowers’ option, either at (i) the Base Rate plus the applicable margin of 3.25% per annum with a stepdown to 3.00% based on Intermediate’s first lien net leverage ratio or (ii) the Adjusted Eurocurrency Rate plus the margin of 4.25% per annum with a stepdown to 4.00% based on Intermediate’s first lien net leverage ratio. The Base Rate is defined as the greatest of (i) the rate last quoted by The Wall Street Journal as the “Prime Rate” in the United States, (ii) the Federal Funds Rate plus 0.50% per annum, (iii) the Adjusted Eurocurrency Rate for a one month interest period plus 1.00% per annum, (iv) solely with respect to the initial term loans, 2.00% per annum and (v) for any loans that are not initial term loans, 1.00% per annum. The “Adjusted Eurocurrency Rate” is defined as the greater of (a) with respect to the initial term loans, the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate (as such term is defined in the First Lien Credit Agreement) and (ii) 1.00% and (b) with respect to the Revolving Credit Facility, the Eurocurrency Rate for such interest period (which, if negative, will be deemed to be 0.00%) multiplied by the Statutory Reserve Rate. The “Eurocurrency Rate” is defined as the London Inter-bank Offered Rate (“LIBOR”) as displayed by Reuters (which, if negative, will be deemed to be 0.00%) or, if LIBOR is unavailable, a rate based on historical LIBOR as determined by the administrative agent under the First Lien Credit Agreement.
As of December 31, 2018 and 2019, the interest rate on the First Lien Term Loan was 6.8125% and 6.0625%, respectively.
Accrued interest under the First Lien Credit Agreement was payable (a) quarterly in arrears with respect to Base Rate loans, (b) at the end of each interest rate period (or at each three-month interval in the case of loans with interest periods greater than three months) with respect to Eurocurrency Rate loans, (c) on the date of any repayment or prepayment and (d) at maturity (whether by acceleration or otherwise). An annual commitment fee was applied to the daily unutilized amount under the Revolving Credit Facility at 0.50% per annum, with one stepdown to 0.375% per annum based on Intermediate’s first lien net leverage ratio.
Borrowings under the Second Lien Credit Agreement bore interest, at the Borrowers’ option, either at (a) the Base Rate plus the applicable margin of 7.00% per annum or (b) the Adjusted Eurocurrency Rate plus the margin of 8.00% per annum. The “Base Rate” is defined as the greatest of (i) the last rate quoted by The Wall Street Journal as the “Prime Rate” in the United States, (ii) the Federal Funds Rate plus 0.50% per annum, (iii) the Adjusted Eurocurrency Rate for a one month interest period plus 1.00% per annum, (iv) solely with respect to the initial term loans, 2.00% per annum and (v) for any loans that are not initial term loans, 1.00% per annum. The “Adjusted Eurocurrency Rate” is defined as (a) with respect to the initial term loans, the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate, and (ii) 1.00% and (b) with respect to the Revolving Credit Facility, the Eurocurrency Rate for such interest period (which, if negative, will be deemed to be 0.0%) multiplied by the Statutory Reserve Rate. The “Eurocurrency Rate” is defined as LIBOR displayed by Reuters (which, if negative, will be deemed to be 0.0%) or, if LIBOR is unavailable, a rate based on historical LIBOR as determined by the administrative agent under the Second Lien Credit Agreement.
As of December 31, 2018 and 2019, the interest rate on the Second Lien Term Loan was 10.37888% and 9.73975%, respectively.
The Borrowers were in compliance with all of their covenants under the First Lien Credit Agreement and Second Lien Credit Agreement as of December 31, 2018 and 2019 and there were no events of default for the year ended December 31, 2019.
New Credit Agreement
On October 19, 2020, Maravai Intermediate Holdings, LLC (“Intermediate”), a wholly-owned subsidiary of ours, along with its subsidiaries Vector Laboratories, TriLink BioTechnologies and Cygnus Technologies (together with Intermediate, the “Borrowers”) entered into the New Credit Agreement with lending institutions, including affiliates of certain of the underwriters, for term-loan borrowings (the “New Term Loan”) totaling $600.0 million to refinance our outstanding senior secured credit facilities and to allow for a distribution to our
120
members. The New Credit Agreement also provided for a revolving credit facility (the “New Revolving Credit Facility”) of $180.0 million for letters of credit and loans to be used for working capital and other general corporate financing purposes. Borrowings under the New Credit Agreement are unconditionally guaranteed by Topco LLC, a wholly owned subsidiary of ours, along with the existing and future material domestic subsidiaries of Topco LLC (subject to certain exceptions) as specified in the respective guaranty agreements, and are secured by a lien and security interest in substantially all of the assets of existing and future material domestic subsidiaries of Topco LLC that are loan parties.
The New Term Loan becomes repayable in quarterly payments of $1.5 million beginning on March 31, 2021, with all remaining outstanding principal due on October 19, 2027. The New Term Loan includes prepayment provisions that allow the Borrowers, at their option, to repay all or a portion of the principal amount at any time. The New Revolving Credit Facility allows the Borrowers to repay and borrow from time to time until October 19, 2025, at which time all amounts borrowed must be repaid. Subject to certain exceptions and limitations, we are required to repay borrowings under the New Term Loan and New Revolving Credit Facility with the proceeds of certain occurrences, such as the incurrence of debt, certain equity contributions, and certain asset sales or dispositions.
Borrowings under the New Credit Agreement bear interest (a) initially, at the Borrowers’ option, either (i) at the Base Rate plus 3.25% per annum or (ii) the Adjusted Eurocurrency Rate plus 4.25% per annum and (b) after delivery of the compliance certificate for the fiscal quarter ending March 31, 2021, at the Borrowers’ option, either at (i) the Base Rate plus the applicable margin of 3.25% per annum with a stepdown to 3.00% based on Intermediate’s first lien net leverage ratio or (ii) the Adjusted Eurocurrency Rate plus the margin of 4.25% per annum with a stepdown to 4.00% based on Intermediate’s first lien net leverage ratio. The Base Rate is defined as the greatest of (i) the rate last quoted by The Wall Street Journal as the “Prime Rate” in the United States, (ii) the NYFRB Rate plus 0.50% per annum, (iii) the Adjusted Eurocurrency Rate for a one month interest period plus 1.00% per annum, (iv) solely with respect to the initial term loans, 2.00% per annum and (v) for any loans that are not initial term loans, 1.00% per annum. The “Adjusted Eurocurrency Rate” is defined as the greater of (a) with respect to the initial term loans the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate (as such term is defined in the New Credit Agreement), and (ii) 1.00% and (b) with respect to the revolving loans, the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate (as such term is defined in the New Credit Agreement), and (ii) 0%. The “Eurocurrency Rate” is defined as the London Inter-bank Offered Rate (“LIBOR”) as displayed by Reuters (which if negative will be deemed to be 0.00%) or, if LIBOR is unavailable, a rate based on historical LIBOR, as determined by the administrative agent under the New Credit Agreement.
Accrued interest under the New Credit Agreement is payable (a) quarterly in arrears with respect to Base Rate loans, (b) at the end of each interest rate period (or at each three-month interval in the case of loans with interest periods greater than three months) with respect to Eurocurrency Rate loans, (c) on the date of any repayment or prepayment and (d) at maturity (whether by acceleration or otherwise). An annual commitment fee is applied to the daily unutilized amount under the New Revolving Credit Facility at 0.375% per annum, with one stepdown to 0.25% per annum based on Intermediate’s first lien net leverage ratio.
Debt Covenants
The New Credit Agreement includes a financial covenant that requires that, if as of the end of any fiscal quarter the aggregate amount of letters of credit obligations and borrowings under the New Revolving Credit Facility outstanding as of the end of such fiscal quarter (excluding cash collateralized letters of credit obligations and letter of credit obligations in an aggregate amount not in excess of $5.0 million at any time outstanding and for the first four fiscal quarters ending after October 19, 2020, borrowings of revolving credit loans made on October 19, 2020) exceed 35% of the aggregate amount of all Revolving Credit Commitments in effect as of such date, then the net leverage ratio of Intermediate shall not be greater than 8.00 to 1.00. For purposes of this covenant, the net leverage ratio is calculated by dividing outstanding first lien indebtedness (net of cash) by Adjusted EBITDA over the preceding four fiscal quarters.
121
The New Credit Agreement also contains negative and affirmative covenants in addition to the financial covenant, including covenants that restrict our ability to, among other things, incur or prepay certain indebtedness, pay dividends or distributions, dispose of assets, engage in mergers and consolidations, make acquisitions or other investments, and make changes in the nature of the business. The New Credit Agreement contains certain events of default, including, without limitation, nonpayment of principal, interest or other obligations, violation of the covenants, insolvency, court ordered judgments, and certain changes of control. The New Credit Agreement also requires Intermediate to provide audited consolidated financial statements to the lenders no later than 120 days after year-end.
The New Credit Agreement also requires mandatory prepayments upon certain excess cash flow, subject to certain step-downs and threshold levels as defined and set forth in the terms of the New Credit Agreement to commence with the fiscal year ending December 31, 2021.
As of October 31, 2020, $600.0 million was outstanding under the New Credit Agreement and the interest rate was 5.25%.
Interest Rate Cap Agreements
As of December 31, 2018 and 2019, we held four interest rate cap agreements and as of September 30, 2020 we held two interest rate cap agreements with a financial institution to manage our variable interest rate risk on a portion of our borrowings under our First Lien Credit Agreement and Second Lien Credit Agreement. As of December 31, 2018 and 2019 and September 30, 2020, the fair value and change in fair value of these interest rate cap agreements was not material.
Cash Flows
The following table summarizes our cash flows for the periods presented:
| Year Ended December 31, |
Nine Months Ended September 30, |
|||||||||||||||
| (in thousands) |
2018 | 2019 | 2019 | 2020 | ||||||||||||
| Net cash (used in) provided by: |
||||||||||||||||
| Operating activities |
$ | (186 | ) | $ | 24,115 | $ | 14,870 | $ | 72,756 | |||||||
| Investing activities |
(3,451 | ) | (17,148 | ) | (12,388 | ) | 14,917 | |||||||||
| Financing activities |
(9,167 | ) | (4,167 | ) | (4,211 | ) | 12,540 | |||||||||
| Effects of exchange rate changes on cash |
(69 | ) | 34 | (21 | ) | (31 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net (decrease) increase in cash |
$ | (12,873 | ) | $ | 2,834 | $ | (1,750 | ) | $ | 100,182 | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
Operating Activities
Net cash provided by operating activities for the nine months ended September 30, 2020 was $72.8 million, which was primarily attributable to a net income of $64.3 million, non-cash depreciation and amortization of $19.9 million, non-cash amortization of deferred financing costs of $1.3 million, non-cash unit-based compensation of $2.9 million, and $2.9 million of acquired in-process research and development, partially offset by a decrease in deferred income taxes of $1.3 million and the gain on a sale and leaseback transaction of $19.0 million.
Net cash provided by operating activities for the nine months ended September 30, 2019 was $14.9 million, which was primarily attributable to a net income of $0.2 million, non-cash depreciation and amortization of $17.4 million, non-cash amortization of deferred financing costs of $1.3 million, non-cash unit-based compensation of $1.2 million, non-cash change in estimated fair value of contingent consideration of $0.2 million, and a net cash outflow from the change in our operating assets and liabilities of $6.1 million. The net change in our operating assets and liabilities reflects a decrease in accounts receivable of $2.6 million, a decrease in prepaid expenses and other current assets of $4.0 million, offset by an increase in accounts payable and accrued liabilities of $0.4 million.
122
Net cash provided by operating activities for the year ended December 31, 2019 was $24.1 million, which was primarily attributable to a net loss of $5.2 million, non-cash depreciation and amortization of $24.1 million, non-cash amortization of deferred financing costs of $1.7 million, non-cash unit-based compensation of $1.7 million, a net cash inflow from the change in our operating assets and liabilities of $2.3 million, partially offset by a decrease in deferred income taxes of $1.2 million. The net change in our operating assets and liabilities reflects a decrease in accounts receivable of $1.9 million, a decrease in prepaid expenses and other current assets of $2.0 million, offset by an increase in accounts payable and accrued liabilities of $6.0 million.
Net cash used in operating activities for the year ended December 31, 2018 was $0.2 million, which was primarily attributable to a net loss of $16.9 million, non-cash depreciation and amortization of $22.3 million, non-cash amortization of deferred financing costs of $1.5 million, non-cash unit-based compensation of $2.1 million, non-cash loss on debt refinancing of $5.6 million, an increase in deferred income taxes of $0.3 million, partially offset by a net cash outflow from the change in our operating assets and liabilities of $15.8 million. The net change in our operating assets and liabilities reflects a decrease of approximately $15.9 million, which was primarily attributable to the payment of an earn-out liability associated with the compensatory cost of a legacy acquisition of a business of $14.5 million.
Investing Activities
Net cash provided by investing activities for the nine months ended September 30, 2020 was $14.9 million, primarily attributable to the proceeds from the sale and leaseback of our Burlingame, California facility of $34.5 million, net of cash outflows of $16.6 million for property and equipment and a cash outflow of $3.0 million associated with the purchase of MockV Solutions, Inc., of which $2.9 million was considered in-process research and development.
Net cash used in investing activities for the nine months ended September 30, 2019 was $12.4 million, which was directly attributable to purchases of property and equipment associated with the buildout of our manufacturing facility in San Diego, California.
Net cash used in investing activities for the year ended December 31, 2019 was $17.1 million, which was primarily attributable to purchases of property and equipment of $17.1 million due to higher capital expenditures related to the buildout of our manufacturing facility in San Diego, California.
Net cash used in investing activities for the year ended December 31, 2018 was $3.5 million, primarily attributable to purchases of property and equipment of $3.5 million.
Financing Activities
Net cash provided by financing activities for the nine months ended September 30, 2020 was $12.5 million, which was primarily attributable to net proceeds of $15.0 million from the drawdown against our revolving line of credit, netted against principal repayment of long-term debt of $1.9 million, distributions to certain holders of MLSC incentive units of $0.3 million and the payment of deferred financing costs of $0.2 million.
Net cash used in financing activities for the nine months ended September 30, 2019 was $4.2 million, which was primarily attributable to principal repayments of long-term debt of $1.9 million and payment of contingent
consideration of $2.0 million, as well as the repurchase of vested incentive units of $0.2 million.
Net cash used in financing activities for the year ended December 31, 2019 was $4.2 million, which was primarily attributable to principal repayments of long-term debt of $2.5 million and payment of contingent consideration of $1.3 million.
Net cash used in financing activities for the year ended December 31, 2018 was $9.2 million, which was primarily attributable to net proceeds of $310.6 million from long term debt borrowings offset by the repayment
123
of the previous long term debt agreement of $255.0 million, a distribution to our member of $52.1 million, financing costs incurred of $6.7 million, and payment of contingent consideration of $5.8 million.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations and commitments as of September 30, 2020:
| Payments due by period | ||||||||||||||||||||
| (in thousands) |
Total | Less than 1 year |
1 to 3 Years |
3 to 5 Years |
More than 5 years |
|||||||||||||||
| Capital lease commitments(1) |
$ | 86 | $ | 12 | $ | 73 | $ | 1 | $ | — | ||||||||||
| Lease facility financing obligations(2) |
45,205 | 560 | 8,438 | 9,426 | 26,781 | |||||||||||||||
| Operating leases(3) |
14,484 | 808 | 5,660 | 2,437 | 5,579 | |||||||||||||||
| Debt obligations(4) |
360,000 | 650 | 5,000 | 5,000 | 349,350 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 419,775 | $ | 2,030 | $ | 19,171 | $ | 16,864 | $ | 381,710 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Represents capital lease commitments. See Note 6 to the interim condensed consolidated financial statements included elsewhere in this prospectus. |
| (2) | Represents lease facility financing obligations. See Note 6 to the interim condensed consolidated financial statements included elsewhere in this prospectus. |
| (3) | Represents operating leases including the ground lease for our San Diego Facility and Southport Facility. See Note 6 to the interim condensed consolidated financial statements included elsewhere in this prospectus. |
| (4) | Represents long-term debt principal maturities, excluding interest. On October 19, 2020, we refinanced our existing $400.0 million credit facilities with a new $780.0 million facility, which is excluded from the amounts presented in this table. See Note 7 and Note 14 to the interim condensed consolidated financial statements included elsewhere in this prospectus. |
The First Lien Credit Agreement required mandatory prepayments upon certain excess cash flow, subject to certain step-downs and threshold levels as defined and set forth in the terms of the First Lien Credit Agreement, commencing with the fiscal year ended December 31, 2019. As of December 31, 2019, the terms of the excess cash flow prepayment provisions did not require any mandatory prepayment.
The New Credit Agreement requires mandatory prepayments upon certain excess cash flow, subject to certain step-downs and threshold levels as defined and set forth in the terms of the New Credit Agreement, to commence with the fiscal year ending December 31, 2021.
Off-Balance Sheet Arrangements
As of September 30, 2020, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Qualitative and Quantitative Disclosures About Market Risk
Interest Rate Risk
As of September 30, 2020, our primary exposure to interest rate risk was associated with our variable rate long-term debt. The First Lien Credit Agreement and Second Lien Credit Agreement bear interest subject to the Base Rate or the Adjusted Eurocurrency Rate. Interest rates can fluctuate for a number of reasons, including changes in the fiscal and monetary policies or geopolitical events or changes in general economic conditions. This could adversely affect our cash flows. We seek to manage these risks through interest rate cap agreements.
124
As of December 31, 2018 and 2019, we had four interest rate cap agreements with a financial institution to manage our variable interest rate risk on a portion of our credit borrowings under the First Lien Credit Agreement and Second Lien Credit Agreement. As of September 30, 2020, two interest rate cap agreements remained outstanding.
We had $360.0 million of outstanding borrowings under our long-term debt facilities as of September 30, 2020. During the years ended December 31, 2018 and, 2019, the effect of a hypothetical 100 basis point increase or decrease in overall interest rates would have changed our interest expense by approximately $3.2 million and $3.5 million, respectively, in each year, and during the nine months ended September 30, 2020 would have changed our interest expense by approximately $2.7 million.
We had cash of $21.9 million, $24.7 million and $124.9 million as of December 31, 2018 and 2019 and September 30, 2020, respectively. Our cash is held in cash demand deposits and is not subject to market risk.
Foreign Currency Risk
The majority of our revenue is denominated in U.S. dollars; however, approximately 40%, 41% and 42% of our revenue was derived from international sales in the years ended December 31, 2018 and 2019 and the nine months ended September 30, 2020, respectively, primarily in Europe and Asia Pacific. However, only our sales in the United Kingdom are denominated in local currency. Our remaining international sales are denominated in the U.S. dollar. Our expenses are generally denominated in the currencies in which they are incurred, which is primarily in the United States and, to a lesser extent, the United Kingdom. As we expand our presence in international markets, to the extent we are required to enter into agreements denominated in a currency other than the U.S. dollar, results of operations and cash flows may increasingly be subject to fluctuations due to changes in foreign currency exchange rates and may be adversely affected in the future due to changes in foreign exchange rates. To date, we have not entered into any hedging arrangements with respect to foreign currency risk. As our international operations grow, we will continue to reassess our approach to manage our risk relating to fluctuations in currency rates.
Critical Accounting Policies and Estimates
We have prepared our consolidated financial statements in accordance with GAAP. Our preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, expenses and related disclosures in the consolidated financial statements. Our estimates are based on historical experience and on various other assumptions that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could differ from these estimates under different assumptions or conditions and any such difference may be material.
While our significant accounting policies are described in more detail in Note 1 to our consolidated financial statements appearing elsewhere in this prospectus, we believe the following discussion addresses our most critical accounting policies, which are those that are most important to our financial condition and results of operations and require our subjective and complex judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We recognize revenue primarily from the sale of manufactured products, including products that can be purchased out of a catalogue and custom manufactured products, and the performance of services, including custom antibody and assay development contracts, antibody affinity extraction and stability and feasibility studies, which often result in the generation of report deliverables. We also have certain licensing and royalty arrangements. Our customers are primarily life science research pharmaceutical and biotechnology companies.
125
We also sell to global and regional distribution partners and original equipment manufacturer (“OEM”) customers who incorporate our products into their products under their own brands.
We adopted the requirements of Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers (“ASC 606”), effective January 1, 2019 using the modified retrospective method. Under the modified retrospective method, this guidance is applied to those contracts that were not completed as of January 1, 2019, with no restatement of contracts that were commenced and completed within fiscal years prior to January 1, 2019, and the prior period comparable financial information continues to be presented under the guidance of ASC 605, Revenue Recognition (“ASC 605”). The adoption of ASC 606 resulted in a cumulative effect adjustment of $0.3 million to reduce the opening accumulated deficit as of January 1, 2019. This adjustment primarily related to over-time recognition of revenue and associated costs for certain products for which revenue had previously been deferred and recognized at a point in time.
Under ASC 606, revenue is recognized when control of promised goods or services is transferred to a customer in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To determine revenue recognition for our arrangements with customers, we perform the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. The majority of our contracts include only one performance obligation. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is defined as the unit of account for revenue recognition under ASC 606. We also recognize revenue from other contracts that may include a combination of products and services, the provision of solely services, or from license fee arrangements which may be associated with the delivery of product. Where there is a combination of products and services, we account for the promises as individual performance obligations if they are concluded to be distinct. Performance obligations are considered distinct if they are both capable of being distinct and distinct within the context of the contract. In determining whether performance obligations meet the criteria for being distinct, we consider a number of factors, such as the degree of interrelation and interdependence between obligations, and whether or not the good or service significantly modifies or transforms another good or service in the contract. As a practical expedient, we do not adjust the transaction price for the effects of a significant financing component if, at contract inception, the period between customer payment and the transfer of goods or services is expected to be one year or less. Contracts with customers are evaluated on a contract-by-contract basis as contracts may include multiple types of goods and services as described below.
Nucleic Acid Production
Nucleic acid production revenue is generated from the manufacture and sale of highly modified, complex nucleic acids products to support the needs of our customers’ research, therapeutic and vaccine programs. The primary offering of products include: CleanCap®, mRNA and oligonucleotide contracts typically consist of a single performance obligation. We also sell nucleic acid products for labeling and detecting proteins in cells and tissue samples research. We recognize revenue from these products in the period in which the performance obligation is satisfied by transferring control to the customer. Revenue for nucleic acid catalog products is recognized at a point in time, generally upon shipment to the customer. Revenue for contracts for certain custom nucleic acid products, with an enforceable right to payment and a reasonable margin for work performed to date, is recognized over time, based on a cost-to-cost input method over the manufacturing period.
Biologics Safety Testing
Our biologics safety testing revenue is associated with the sale of bioprocess impurity detection kit products. We also enter into contracts that include custom antibody development, assay development and antibody affinity extraction services. These products and services enable the detection of impurities and contaminants that occur in the manufacturing of biologic drugs and other therapeutics. We recognize revenue from the sale of bioprocess
126
impurity detection kits in the period in which the performance obligation is satisfied by transferring control to the customer. Custom antibody development contracts consist of a single performance obligation, typically with an enforceable right to payment and a reasonable margin for work performed to date. Revenue is recognized utilizing a cost-based input method over the term of the contract. Where an enforceable right to payment does not exist, revenue is recognized at a point in time when control is transferred to the customer. Assay development service contracts, which generally occur over a short period of time and consist of a single performance obligation, revenue is recognized at a point in time when a successful antigen test and report is provided to the customer. Affinity extraction services consist of a single performance obligation to perform the extraction service and provide a summary report to the customer. Revenue is recognized either over time or at a point in time depending on contractual payment terms with the customer.
Protein Detection
We also manufacture and sell protein labeling and detection reagents used by researchers in protein labeling and detection. The contracts to sell these catalog products consist of a single performance obligation to deliver the reagent products. Revenue from these contracts is recognized at a point in time, generally upon shipment of the product to the customer.
We recognize royalty revenue related to certain out-licensing and royalty arrangements in the period the sales or usage occur using third-party evidence to estimate the amount to be recorded. To date this revenue has not been material to the consolidated financial statements.
We have elected the practical expedient to not disclose the unfulfilled performance obligations for contracts with an original length of one year or less. We had no material unfulfilled performance obligations for contracts with an original length greater than one year as of December 31, 2019.
We accept returns only if the products do not meet customer specifications and historically, our volume of product returns has not been significant. Further, no warranties are provided for promised goods and services other than assurance type warranties.
Revenue for an individual contract is recognized at the related transaction price, which is the amount we expect to be entitled to in exchange for transferring the products and/or services. The transaction price for product sales, which excludes sales taxes we collect, is calculated at the contracted product selling price. The transaction price for a contract with multiple performance obligations is allocated to the separate performance obligations on a relative standalone selling price basis. Standalone selling prices for products are determined based on the prices charged to customers, which are directly observable. Standalone selling price of services are mostly based on time and materials. Generally, payments from customers are due when goods and services are transferred. As most contracts contain a single performance obligation, the transaction price is representative of the standalone selling price charged to customers. Revenue is recognized only to the extent that it is probable that a significant reversal of the cumulative amount recognized will not occur in future periods. Since the adoption of ASC 606, variable consideration has not been material. For arrangements where the anticipated period between timing of transfer of goods and services and the timing of payment is one year or less, we have elected to not assess whether a significant financing component exists.
We have elected to account for shipping and handling activities related to contracts with customers as costs to fulfill the promise to transfer the associated products. Accordingly, revenue for shipping and handling is recognized at the same time that the related product revenue is recognized.
Contract assets are generated when contractual billing schedules differ from revenue recognition timing and we record contract receivable when it has an unconditional right to consideration. Contract liabilities are recorded when cash payments are received or due in advance of performance.
127
Applying the practical expedient, we recognize the incremental costs of obtaining contracts as an expense when incurred when the amortization period of the assets that otherwise would have been recognized is one year or less. These costs are included in sales and marketing and general and administrative expenses. The costs to fulfill the contracts are determined to be immaterial and are recognized as an expense when incurred.
Prior to January 1, 2019, revenue from the sale of products and services was recognized when all of the following conditions per ASC 605, Revenue Recognition were met: (1) there was persuasive evidence of an arrangement; (2) the product or service had been delivered to the customer; (3) the collection of the fees was reasonably assured; and (4) the amount of fees to be paid by the customer was fixed or determinable.
When an arrangement involved multiple elements, the multiple elements, referred to as deliverables, were evaluated to determine whether they represent separate units of accounting in accordance with ASC 605-25, Revenue Recognition—Multiple-Element Arrangements. We performed this evaluation at the inception of an arrangement and as each item was delivered in the arrangement. Generally, we accounted for a deliverable separately if the delivered item has standalone value to the customer and delivery or performance of the undelivered item or service was probable and substantially in our control.
When multiple elements could be separated into separate units of accounting, arrangement consideration was allocated at the inception of the arrangement, based on each unit’s relative selling price, and recognized based on the method most appropriate for that unit.
Inventory
Our inventories consist of raw materials, work in process and finished goods. Inventories are stated at the lower of cost (weighted average cost) or net realizable value. Inventory costs include materials, direct labor and manufacturing overhead, which are related to the purchase or production of inventories. We review our inventory at least quarterly for excess and obsolete inventory based on our estimates of expected sales volumes, production capacity and expiration of raw materials, work-in-process and finished products. Expected sales volumes are determined based on internal sales forecasts that consider both historical and projected sales. We write down inventory that has become obsolete, inventory that has a cost basis in excess of its expected net realizable value, and inventory in excess of expected manufacturing requirements. Any write-downs of inventories are charged to cost of revenue.
A change in the estimated timing or amount of demand for our products could result in additional provisions for excess inventory quantities on hand. Any significant unanticipated changes in demand or unexpected quality failures could have a significant impact on the value of inventory and reported operating results. During all periods presented in the accompanying consolidated financial statements, there have been no material adjustments related to a revised estimate of inventory valuations.
Goodwill
Goodwill represents the excess of consideration transferred over the estimated fair value of assets acquired and liabilities assumed in a business combination. We conduct a goodwill impairment analysis in the fourth quarter of each year, and more frequently if changes in facts and circumstances indicate that the fair value of our reporting units may be less than carrying amount. To analyze goodwill for impairment, we must assign our goodwill to individual reporting units. Identification of reporting units includes an analysis of the components that comprise each of our operating segments, which considers, among other things, the manner in which we operate our business and the availability of discrete financial information. Components of an operating segment are aggregated to form one reporting unit if the components have similar economic characteristics. We periodically review our reporting units to ensure that they continue to reflect the manner in which we operate our business.
128
Accounting guidance also permits an optional qualitative assessment for goodwill on a reporting unit by reporting unit basis to determine whether it is more likely than not that the carrying value of a reporting unit exceeds its fair value. If, after this qualitative assessment, we determine that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, then no further quantitative testing would be necessary. A quantitative assessment is performed if the qualitative assessment results in a more likely than not determination or if a qualitative assessment is not performed. The quantitative assessment considers whether the carrying amount of a reporting unit exceeds its fair value, in which case an impairment charge is recorded to the extent the reporting unit’s carrying value exceeds its fair value.
As of December 31, 2018 and 2019, we concluded that we operated as three reporting units and elected to perform a qualitative impairment test for our nucleic acid production and biologics safety testing reporting units and a quantitative impairment test for our protein detection reporting unit. The qualitative impairment test was elected for the nucleic acid production and biologics safety testing reporting units because of the growth in revenue and cash flows in excess of our initial projections and the quantitative impairment test was elected for the protein detection reporting unit as a result of lowering forecasted growth projections. The fair value of the protein detection reporting unit was determined using both an income approach and market approach. The income approach is a valuation technique under which we estimated future cash flows using the protein detection reporting unit’s financial forecast from the perspective of an unrelated market participant. Using historical trending and internal forecasting techniques, we projected revenue and applied our fixed and variable cost experience rates to the projected revenue to arrive at the future cash flows. A terminal value was then applied to the projected cash flow stream. Future estimated cash flows were discounted to their present value to calculate the estimated fair value. The discount rate used was the value-weighted average of our estimated cost of capital derived using both known and estimated customary market metrics. In determining the estimated fair value of the protein detection reporting unit, we were required to estimate a number of factors, including projected operating results, terminal growth rates, economic conditions, anticipated future cash flows, the discount rate and the allocation of shared or corporate items. Our market approach model estimates the fair value of the protein detection reporting unit based on market prices paid in actual precedent transactions of similar businesses and market multiples of guideline public companies. This impairment assessment is sensitive to changes in forecasted cash flows, as well as our selected discount rate. Changes in the protein detection reporting unit’s results, forecast assumptions and estimates could materially affect the estimation of its fair value.
As a result of our 2018 and 2019 qualitative and quantitative assessments, we concluded that goodwill was not impaired as of December 31, 2018 or 2019.
Valuation of Intangible Assets
In conjunction with our business combinations, we have recorded intangible assets primarily consisting of trade names, customer relationships, patents, and developed technology. Certain criteria are used in determining whether intangible assets acquired in a business combination must be recognized and reported separately. Finite-lived intangible assets are initially recognized at fair value, are subject to amortization and are subsequently stated at amortized cost. Our finite-lived intangible assets are amortized using a method that reflects the pattern in which the economic benefits of the intangible assets are consumed or otherwise used. If that pattern cannot be reliably determined, the intangible assets are amortized using the straight-line method over their estimated useful lives.
We periodically review our finite-lived intangible assets, to determine whether current events or circumstances indicate that such carrying amounts may not be recoverable. If such facts or circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets is compared to the carrying value the assets to determine whether impairment exists. If the assets are determined to be impaired, the loss is measured based on the difference between the fair value and carrying value of the assets. No impairment loss was recognized for finite-lived intangible assets during the years ended either December 31, 2018 or 2019.
129
Leases, Deferred Rent and Lease Facility Financing Accounting
We rent our office space and facilities under non-cancelable operating lease agreements and recognize related rent expense on a straight-line basis over the term of the lease. Our lease agreements contain rent holidays, scheduled rent increases and renewal options. Rent holidays and scheduled rent increases are included in the determination of rent expense to be recorded ratably over the lease term. We do not assume renewals in its determination of the lease term unless they are deemed to be reasonably assured at the inception of the lease. We begin recognizing rent expense on the date that we obtain the legal right to use and control the leased space. Deferred rent consists of the difference between cash payments and the recognition of rent expense on a straight-line basis for the buildings we occupy.
Funding of leasehold improvements by our landlord is accounted for as a tenant improvement allowance and recorded as current and non-current deferred rent liabilities and amortized on a straight-line basis as a reduction of rent expense over the term of the lease.
In certain arrangements, we are involved in the construction of improvements to buildings we are leasing. To the extent we are involved with the structural improvements of the construction project or take construction risk, we are considered to be the owner of the building and related improvements for accounting purposes during the construction period. Therefore, we record the fair value of the building subject to the lease within property and equipment on the balance sheet, plus the amount of building improvements incurred and funded by us and/or the landlord as of the balance sheet date. We also record a corresponding lease financing obligation on our balance sheet representing the amounts financed by the lessor for the building and lessor financed improvements. Lessor financed improvement incentives due but not yet received are recorded as prepaid expense and other current assets on the balance sheet.
Once construction is completed, we consider the requirements for sale-leaseback accounting treatment, including evaluating whether all risks of ownership have been transferred back to the landlord, as evidenced by a lack of our continuing involvement in the leased property. If we conclude the arrangement does not qualify for sale-leaseback accounting treatment, the building and improvements remain on our balance sheet and are subject to depreciation and assessment of impairment. We bifurcate our lease payments into a portion allocated to the lease financing obligation and a portion allocated to the parcel of land on which the building has been built. The portion of the lease payments allocated to the land is treated for accounting purposes as operating lease payments, and therefore is recorded as rent expense in the consolidated statements of operations and comprehensive loss. The portion of the lease payments allocated to the lease financing obligation is further bifurcated into a portion allocated to interest expense and a portion allocated to reduce the lease financing obligation.
The interest rate used for the lease financing obligation represents our estimated incremental borrowing rate at the inception of the lease, adjusted to reduce any built-in loss. The initial recording of these assets and liabilities is classified as non-cash investing and financing items, respectively, for purpose of the consolidated statements of cash flows.
The most significant estimates used by us in accounting for the lease financing transaction and the impact of these estimates are as follows:
| • | Incremental borrowing rate. We estimate our incremental borrowing rate as the rate we would have incurred to borrow, based on our credit quality at the inception of the lease over a similar term, the funds necessary to purchase the leased building subject to the financing lease transaction. The incremental borrowing rate is used in determining allocating our rental payments between interest expense and a reduction of the outstanding lease financing obligation. |
| • | Land capitalization rate. The land capitalization rate is the rate of return on the land underlying the lease properly considering expected income that the land would be expected to generate. The land lease |
130
| capitalization rate is estimated using comparable market data for land capitalization rates for similar properties. The land capitalization rate is used in determining allocating our rental payments between interest expense and a reduction of the outstanding lease financing obligation. |
| • | Fair value of leased building and underlying land. The fair value of a leased building and underlying land subject to the lease financing transaction is based on comparable market data for similar properties as of the lease inception date. The fair value of the underlying land is used in determining allocating our rental payments between interest expense and a reduction of the outstanding lease financing obligation. |
Prior to our October 2016 acquisition of MLSC, the Southport, North Carolina facility (the “Southport Facility”) leased by a subsidiary of MLSC failed to qualify for sale and leaseback accounting. As a result, MLSC recognized during construction, and retained upon the completion of construction, the value of the Southport Facility and obligation on its balance sheet as a financing obligation. Pursuant to the business combination fair value guidance, upon acquisition of MLSC, we recorded the fair value of the building asset, which was estimated to be $2.2 million, and the related financing obligation of $2.2 million. We continue to recognize payments under the amended lease agreement as a reduction of the facility financing obligation using the effective interest method and the ground rent as operating lease expense. At the conclusion of the lease term, we will de-recognize both the then carrying values of the asset and financing obligation with any differences between the book value of the building asset and remaining facility financing obligation being recognized in operations at that time. For its existing arrangement for the Southport Facility, these differences are expected to be immaterial.
In July 2018, we entered into a non-cancelable lease for a new manufacturing facility (the “San Diego Facility Lease”) and subsequently took possession of the space. The scope of the tenant improvements did not qualify under the lease accounting guidance as “normal tenant improvements” and we were the deemed owner of the leased building during the construction period for accounting purposes. In 2019, construction on the facility was substantially completed and the leased property was placed into service. We determined that the completed construction project did not qualify for sale-leaseback accounting due to non-recourse financing we provided to the lessor for reimbursed construction costs and has instead been accounted for as a financing transaction. The leased building for the San Diego Facility Lease and related improvements remains on our consolidated balance sheet as of December 31, 2019 and rental payments associated with the lease have been allocated to operating lease expense for the ground underlying the leased building and principal and interest payments on the lease financing obligation.
Unit-Based Compensation and Incentive Unit Valuation
Unit-based awards have been granted by our parent and also by one of our subsidiaries to certain executives and employees of our subsidiaries in the form of non-vested incentive units (“Incentive Units”). All awards of Incentive Units are measured based on the fair value of the award on the date of grant. We recognize compensation expense for these awards over the requisite service period. Forfeitures are recognized when they occur. Unit-based compensation expenses are classified in the consolidated statements of operations based on the job functions of the related employees. These Incentive Units are subject to service, market or performance conditions. For Incentive Units subject to performance conditions, we evaluate the probability of achieving each performance condition at each reporting date and recognize expense over the requisite service period when it is deemed probable that a performance condition will be met using the accelerated attribution method over the requisite service period. For Incentive Units that remain subject to performance conditions as of December 31, 2018 and 2019, we concluded that it was not yet probable that the performance conditions would be met. Accordingly, we have not recognized any compensation expense loss for Incentive Units that include a performance condition.
As there has been no public market for the Incentive Units granted by our parent or by our subsidiary, the grant date fair value of Incentive Unit awards has been determined by our board of directors with the assistance of management and an independent third-party valuation specialist. We believe our board of directors has the
131
relevant experience and expertise to determine the fair value of our Incentive Units. The grant date fair value of Incentive Units was determined first by estimating our aggregate equity value using a weighting of discounted cash flows, comparable public companies, and comparable-transactions valuation methodologies. An Option-Pricing Method, which utilizes certain assumptions including volatility, time to liquidation, a risk-free interest rate, and an assumption for a discount for lack of marketability, was then used to allocate our total equity value to our different classes of equity according to their rights and preferences. A discount for lack of marketability was applied to determine the Incentive Unit equity values. In determining the fair value of the Incentive Units, the methodologies used to estimate our enterprise value were performed using methodologies, approaches, and assumptions consistent with the American Institute of Certified Public Accountants Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation (“AICPA Accounting and Valuation Guide”). The assumptions we use in the valuation model are based on future expectations combined with management’s judgment. In the absence of a public trading market, our board of directors, with input from management, exercised significant judgment and considered numerous objective and subjective factors to determine the fair value of the Incentive Units as of the date of each award, including the following factors:
| • | independent valuations performed at periodic intervals by an independent third-party valuation firm; |
| • | our operating and financial performance, forecasts and capital resources; |
| • | current business conditions; |
| • | the hiring of key personnel; |
| • | the status of research and development efforts; |
| • | the likelihood of achieving a liquidity event for these incentive units, such as an initial public offering or sale of our company, given prevailing market conditions; |
| • | any adjustment necessary to recognize a lack of marketability for the Incentive Units; |
| • | trends and developments in our industry; |
| • | the market performance of comparable publicly traded technology companies; and |
| • | the U.S. and global economic and capital market conditions. |
The dates of our valuation reports, which were prepared on a periodic basis, were not contemporaneous with the grant dates of our Incentive Unit awards. Therefore, we considered the amount of time between the valuation report date and the grant date to determine whether to use the latest Incentive Unit valuation report for the purposes of determining the fair value of our units for financial reporting purposes. If Incentive Units were granted a short period of time preceding the date of a valuation report, we assessed the fair value of such Incentive Unit award used for financial reporting purposes after considering the fair value reflected in the subsequent valuation report and other facts and circumstances on the date of grant as described below. The additional factors considered when determining any changes in fair value between the most recent valuation report and the grant dates included, when available, the prices paid in recent transactions involving our Incentive Units, as well as our operating and financial performance, current industry conditions and the market performance of comparable publicly traded companies. There were significant judgments and estimates inherent in these valuations, which included assumptions regarding our future operating performance, the time to completing an initial public offering or other liquidity event and the determinations of the appropriate valuation methods to be applied. If we had made different estimates or assumptions, our unit-based compensation expense, net loss and net loss per unit attributable to our member could have been significantly different from those reported in this prospectus.
In valuing our units, the executive committee determined the equity value of our business by taking a weighted combination of the value indications using the income approach and the market comparable approach valuation methods.
132
Income Approach
The income approach estimates value based on the expectation of future cash flows a company will generate, such as cash earnings, cost savings, tax deductions and the proceeds from disposition. These future cash flows are discounted to their present values using a discount rate derived from an analysis of the cost of capital of comparable publicly traded companies in our industry or similar lines of business as of each valuation date. This weighted-average cost of capital discount rate, or WACC, is adjusted to reflect the risks inherent in the business. The WACC used for these valuations was determined to be reasonable and appropriate given our debt and equity capitalization structure at the time of each respective valuation. The income approach also assesses the residual value beyond the forecast period and is determined by taking the projected residual cash flow for the final year of the projection and applying a terminal exit multiple. This amount is then discounted by the WACC less the long-term growth rate.
Market Comparable Approach
The market comparable approach estimates value based on a comparison of the subject company to comparable public companies in a similar line of business. From the comparable companies, a representative market multiple is determined which is applied to our financial metrics to estimate the value of our parent or our subsidiary. To determine our peer group of companies, we considered life sciences tools companies and selected those most similar to us based on various factors, including, but not limited to, financial risk, company size, geographic diversification, profitability, growth characteristics and stage of life cycle.
In some cases, we considered the amount of time between the valuation date and the award grant date to determine whether to use the latest valuation determined pursuant to one of the methods described above or to use a valuation calculated by management between the two valuation dates.
In some cases, we considered the amount of time between the valuation date and the award grant date to determine whether to use the latest valuation determined pursuant to one of the methods described above or to use a valuation calculated by management between the two valuation dates.
Once we determined an equity value, we utilized the Black-Scholes Option Pricing Model (“BSOPM”) to allocate the equity value to our options. BSOPM values our options by creating call options on the respective equity value, with exercise prices based on the liquidation preferences, participation rights and strike prices. This method is generally preferred when future outcomes are difficult to predict and dissolution or liquidation is not imminent.
As of September 30, 2020, the aggregate value of our vested and unvested Incentive Units was $8.0 million and $9.0 million, respectively, based on the estimated fair value for one unit of $25.50 per share of Class A common stock, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus. As of September 30, 2020, we had $4.1 million of unrecognized compensation expense related to Incentive Units not subject to a performance condition that is expected to be recognized over a weighted-average period of 3.6 years. For Incentive Units subject to a performance condition, unrecognized compensation expense was $3.9 million as of September 30, 2020.
Income Taxes
Our wholly-owned U.S. subsidiary, Maravai Life Sciences, Inc. (“Maravai Inc.”) and its subsidiaries, are taxpaying entities in the U.S., Canada, and the U.K, and we provide current and deferred income taxes for these entities. We and our other subsidiaries are treated as pass-through entities for federal and state income tax purposes. The income or loss generated by these entities is not taxed at the LLC level. As required by U.S. tax law, income or loss generated by these LLCs passes through to their owners. As such, our tax provision consists solely of the activities of Maravai Inc. and its subsidiaries, which is taxed as a corporation for federal and state income tax purposes.
133
Our taxable subsidiaries account for income taxes under the asset and liability method of accounting. We recognize deferred tax assets and liabilities for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, as well as for operating loss and tax credit carryforwards. We measure deferred tax assets and liabilities using enacted tax rates expected to apply to taxable income in the years in which we expect to recover or settle those temporary differences. We recognize the effect of a change in tax rates on deferred tax assets and liabilities in the results of operations in the period that includes the enactment date. We reduce the measurement of a deferred tax asset, if necessary, by a valuation allowance if it is more likely than not that we will not realize some or all of the deferred tax asset.
We account for uncertain tax positions by recognizing the financial statement effects of a tax position only when, based upon technical merits, it is more likely than not that the position will be sustained upon examination.
Significant judgment is required in determining the accounting for income taxes. In the ordinary course of business, many transactions and calculations arise where the ultimate tax outcome is uncertain. Our judgments, assumptions and estimates relative to the accounting for income taxes take into account current tax laws, our interpretation of current tax laws, and possible outcomes of future audits conducted by foreign and domestic tax authorities. Although we believe that our estimates are reasonable, the final tax outcome of matters could be different from our assumptions and estimates used when determining the accounting for income taxes. Such differences, if identified in future periods, could have a material effect on the amounts recorded in our consolidated financial statements.
JOBS Act Accounting Election
We are an “emerging growth company” within the meaning of the JOBS Act. The JOBS Act permits an emerging growth company like us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are electing to use this extended transition period and we will therefore comply with new or revised accounting standards when they apply to private companies. As a result, our financial statements may not be comparable with companies that comply with public company effective dates for accounting standards. We also intend to rely on other exemptions provided by the JOBS Act, including not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act.
We will remain an emerging growth company until the earliest of (1) December 31, 2025, (2) the last day of the fiscal year in which we have total annual gross revenue of at least $1.07 billion, (3) the last day of the fiscal year in which we are deemed to be a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock held by non-affiliates exceeded $700.0 million as of the last business day of the second fiscal quarter of such year or (4) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
Recent Accounting Pronouncements
See Note 1 to our audited consolidated financial statements included elsewhere in this prospectus for more information.
134
Overview
We are a leading life sciences company providing critical products to enable the development of drug therapies, diagnostics, novel vaccines and support research on human diseases. Our more than 5,000 customers as of September 30, 2020 include the top 20 global biopharmaceutical companies ranked by research and development expenditures according to industry consultants, and many other emerging biopharmaceutical and life sciences research companies, as well as leading academic research institutions and in vitro diagnostics companies. Our products address the key phases of biopharmaceutical development and include complex nucleic acids for diagnostic and therapeutic applications, antibody-based products to detect impurities during the production of biopharmaceutical products, and products to detect the expression of proteins in tissues of various species.

Our businesses principally address high growth market segments in biopharmaceutical development and manufacturing. We estimate that the market segments we serve are growing at a weighted average blended rate of 20% per annum. In particular, the field of cell and gene therapy has emerged as one of the fastest growing treatment modalities to address a host of human conditions. There are more than 400 cell and gene therapies in development or launched and sales in this category are expected to grow more than tenfold by 2024, according to industry consultants and management estimates. Our portfolio offers key products for each stage of the cell and gene therapy development lifecycle. For example, our mRNA products are used in drug development to assist in the production of immune-activating antigens; our CleanCap® technology is used to stabilize mRNA; and we expect our upcoming plasmid DNA products will be used as templates for the production of our RNA products. We also provide biologics safety testing technology used to ensure the safety of the biological drug manufacturing process and drug products. We estimate that more than 64% of our revenue for the nine months ended September 30, 2020 was in support of vaccines and therapies in development, including biological drugs and cell and gene therapies. We have relationships with the following categories of customers (percentages represent the share of revenue in each category for the nine months ended September 30, 2020): developers of therapeutics and vaccines (63%), other biopharmaceutical and life science research companies (29%), academic institutions (5%) and molecular diagnostic companies (3%).
Our proprietary capabilities and products underpin the value we aim to provide to our customers. Among other capabilities, we are expert in RNA and mRNA products, which are challenging and often unstable molecules requiring significant chemical modifications to ensure their stability and efficacy in our customers’ applications. Notably, according to research commissioned by us consisting of over 70 interviews with our current and former customers, our competitors and industry experts focused across our three business segments (the “Industry Analysis”), we believe CleanCap® is viewed as a leading solution to ensure the stability of mRNA. CleanCap® is a novel chemical approach to produce a cap analog, which, in addition to making mRNA more stable, aids in protein production and helps prevent an unwanted immune response to the mRNA. As of
135
September 30, 2020, CleanCap® had been used by over 100 customers and had been incorporated into several development programs targeting immunization against the novel strain of coronavirus, SARS-CoV-2 (“COVID-19”). These programs included one phase II/III clinical program led by Pfizer in partnership with BioNTech, three phase I/II clinical programs led by Imperial College London, Fosun Pharma in partnership with BioNTech and CureVac and one pre-clinical program led by the University of Tokyo in partnership with Daiichi-Sankyo. In addition, CleanCap® is currently being used in three additional COVID-19 mRNA vaccine programs that are in earlier stages of development, led by Chula Vaccine Research Center in partnership with the University of Pennsylvania, eTheRNA Immunotherapies and Greenlight Biosciences. Given the early stage of these three programs, there can be no assurance they will continue to use CleanCap® through commercialization. We estimate our mRNA and CleanCap® products have also been incorporated in at least 33 therapeutic programs in development as of September 30, 2020. These therapeutic programs address a number of disease states, including ornithine transcarbamylase deficiency, glycogen storage disorders, Alpha-1 antitrypsin deficiency, acute lymphoblastic leukemia, Hurler syndrome, ovarian cancer and cardiovascular disease. These therapeutic programs also use multiple therapeutic modalities, including CRISPR/Cas-9, transcription activator-like effector nuclease (TALENS), enzyme replacement therapies, allogeneic CAR-T cells and base editing. Should one or more of these programs proceed to commercialization, we believe we will continue to supply our customers and our products will likely be incorporated in customer regulatory filings.
mRNA is at the core of our capabilities. We developed our expertise in mRNA with a belief in its potential as a therapeutic modality. The first clinical trial for an mRNA therapeutic agent occurred in 2016. More than 30 clinical trials have occurred since then, principally focused on vaccines against viruses and cancer vaccines. With the COVID-19 pandemic, mRNA has shown its potential for more rapid vaccine design and manufacture when compared to traditional techniques involving culturing inactivated virus to elicit an immune response. According to the World Health Organization, there were 202 COVID-19 vaccine development programs as of November 3, 2020, with some lead candidates for approval in the RNA class and anticipated data readouts from certain RNA programs, including results of preclinical studies and phase I/II and phase III clinical trials. COVID-19 has helped highlight the potential advantage of mRNA as a treatment modality and directed significant resources to the developing base of knowledge about mRNA. We believe this knowledge will be directed at future vaccine programs as well as therapeutic agents for a host of human diseases. We are positioned to serve our biopharmaceutical customers in the fast-growing mRNA field across a range of clinical programs for a variety of diseases. Approximately 39% of our revenue was derived from products that support mRNA research for the nine months ended September 30, 2020.
Forming long-term partnerships with our customers is core to our strategy. We primarily serve our customers during the product development and process development phases. During product development, we collaborate with our customers to develop and synthesize nucleic acids, which in some cases comprise the APIs of our customers’ products in development. We also provide our customers a host of chemically complex and highly specialized raw materials. Process development entails establishing production methods in anticipation of regulatory approval of biopharmaceutical products. Process development is a complex phase that establishes highly validated procedures and determines the investment in facilities and equipment required to bring biopharmaceutical products to market. These decisions impact the viability of our customers’ products for the long term. During process development, we provide enzyme-linked immunosorbent assays (“ELISAs”) that reduce the risk posed by impurities and contaminants in biological drugs, a critical step to ensure the safety of the drug product.
While we do not provide products that are themselves regulated as drugs or in vitro diagnostics, our customers frequently incorporate our products into their highly validated products and processes. For example, we provide oligonucleotides and antibody-based products used by in vitro diagnostic product manufacturers for their on-market products. Because of the extensive validation required for these products, these components are frequently purchased for the life of our customers’ products and we believe they are unlikely to be substituted. In addition, our analytical tools are used in the design and development of manufacturing processes and often will be used throughout the life cycle of our customers’ manufactured products. As a result, our customer relationships may span many years.
136
The nature of our products and their uses require that they be manufactured by highly trained personnel in state-of-the-art facilities following exacting procedures to ensure quality. We manufacture our nucleic acid products at our San Diego, California facility, one of four facilities we occupy in the United States. The facility was purpose-built to address our customers’ needs for critical raw materials manufactured under certain good manufacturing practices (“GMP”) conditions and APIs for investigational use. Our raw material products are manufactured following the voluntary quality standards of ISO 9001:2015. Our GMP-grade raw materials follow ISO 9001:2015 standards, additional voluntary GMP quality standards and customer specific requirements. Our API products are manufactured following the voluntary quality standards of ISO 9001:2015, the International Council for Harmonisation’s GMP Guide, comparable GMP principles for the European Union and customer specific requirements. We believe our products are exempt from compliance with the current GMP (“cGMP”) regulations of the Food and Drug Administration (“FDA”), as our products are further processed or incorporated into final drug products by our customers and we do not make claims related to their safety or effectiveness. As of September 30, 2020, we estimate that $70 million has been invested in our San Diego facility. Our other facilities are similarly designed for specific applications with quality systems to match our customers’ requirements. All of our facilities meet applicable ISO standards. In addition, as of September 30, 2020, approximately 17% of our workforce have earned advanced degrees and all receive rigorous on the job training.
We built our business through a combination of acquisitions and subsequent investments in our acquired companies to grow their commercial capabilities, upgrade and expand their research and production facilities, deploy stringent quality systems, integrate their back-office functions, and develop the personnel and management to fuel continued growth. Today, we offer an integrated portfolio that enables innovation across the biopharmaceutical and academic markets. We completed our first acquisition in April 2016. The trailing twelve-month revenue of each of our acquired businesses at the time we acquired each of them totaled approximately $85.0 million. Mergers, acquisitions and strategic partnerships that complement our capabilities in cell and gene therapy and biopharmaceutical production remain core to our strategy. Our strategy aims to augment our strong organic growth with the addition of synergistic products and capabilities.
Our Portfolio and Capabilities
We provide products that support our customers’ needs from discovery through commercialization of their vaccines, therapeutic agents and in vitro diagnostic products. Our products are frequently incorporated into our customers’ products, whether as research products or APIs used in development or research products incorporated as raw materials into on-market products. They may also be incorporated into the manufacturing process itself. We are therefore a critical part of our customers’ supply chain and they frequently seek to maintain their supply relationship with us for the life of their products or development programs.
137
Our products address our customers’ needs for nucleic acid production, biologics safety testing and protein detection, and our operations are aligned to these three segments. For the nine months ended September 30, 2020, we sold more than 53% of our products and services to biopharmaceutical customers and our products serve high growth applications in vaccines, cell and gene therapies, biological drugs and molecular diagnostics.
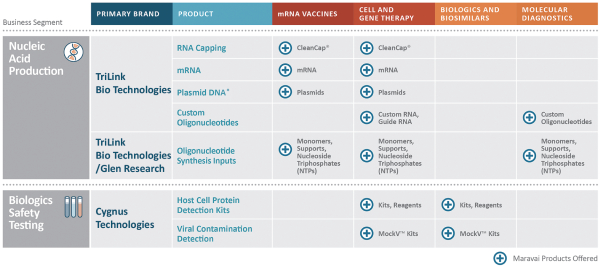
| * | Our plasmid DNA products are expected to launch in Q1 2021 |
Nucleic Acid Production (69% of Revenue for the Nine Months Ended September 30, 2020)
We are a global provider of highly modified, complex nucleic acids and related products. We have recognized expertise in complex chemistries and products provided under exacting quality standards. Our core offerings include mRNA, long and short oligonucleotides, our proprietary CleanCap® capping technology and oligonucleotide building blocks. Our offerings address key customer needs for critical components, from research to GMP-grade materials. We market our nucleic acid products under the TriLink BioTechnologies and Glen Research brands.
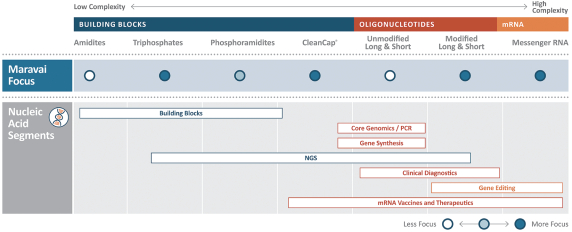
The growth in our nucleic acid production business segment has been fueled by the significant growth in biological drugs in development, many of which are addressing cell and gene therapies, and by the rapid rise in mRNA vaccines. mRNA as a treatment modality has been an area of acute interest for several years. The global
138
COVID-19 pandemic, however, has highlighted its potential advantages in speed of development and manufacturing, as well as cost. Of the estimated 202 COVID-19 vaccine development programs underway as of November 3, 2020, according to the World Health Organization, 26 are mRNA-based. Five of the 26, including one phase II/III clinical program led by Pfizer in partnership with BioNTech, three phase I/II clinical programs led by Imperial College London, Fosun Pharma in partnership with BioNTech and CureVac and one pre-clinical program led by the University of Tokyo in partnership with Daiichi-Sankyo, use our CleanCap® products and up to three more in earlier stages of development, led by Chula Vaccine Research Center in partnership with the University of Pennsylvania, eTheRNA Immunotherapies and Greenlight Biosciences, are currently using our CleanCap® products. Given the early stage of these three programs, there can be no assurance they will continue to use CleanCap® through commercialization. We further serve cell and gene therapies with our RNA and mRNA products and expect to supplement with our upcoming plasmid DNA capability. In addition to the vaccine programs above, our products have been incorporated in 33 therapeutic programs in development for CRISPR/Cas-9, CAR-T, base editing and enzyme replacement therapies, among others.
Our nucleic acid products fall into four broad categories: CleanCap®, mRNA, oligonucleotides and plasmid DNA. We expect to offer our plasmid DNA products in the first quarter of 2021.
CleanCap®. Our proprietary CleanCap® analogs principally serve the mRNA vaccine and therapeutic markets, including vaccine candidates in development for immunizing against COVID-19. Cap analogs are a component of mRNA that aids in protein production as well as making mRNA more stable inside cells. For mRNA to serve as a template to make a protein, it requires a special cap at the 5’ (five prime) end of the molecule. The cap structure also affects the stability of the mRNA. The lack of a cap can result in activation of the innate immune system, which can affect the production of the desired protein or elicit undesired biological effects. We offer a suite of CleanCap® analogs that are specifically made for therapeutics and vaccines. Based on the Industry Analysis, we believe our cap analogs are critical features of several mRNA vaccines in development.
|
|
|
CleanCap® is a synthetic capping reagent composed of N7 Methyl (G) linked to a dimer at (A) and (G) through a triphosphate (P) linkage that is added during the transcription reaction and resulting in high levels of mRNA capping.
|
Traditionally, the 5’ cap has been added in one of two ways. The cap can be added post mRNA synthesis by an enzymatic process. This enzymatic method has several drawbacks, including the high cost of the capping enzymes as well as the need to perform additional processing steps to the mRNA to remove enzymes and byproducts of the capping reaction. While capping efficiency is usually high, the extra processing steps typically result in mRNA of poorer quality and degradation often results. The second method is to add a synthetic cap analog into the transcription reaction such that the mRNA is transcribed and capped in a single step. Anti-reverse cap analog (“ARCA”) is an example of a cap analog that is added to the transcription reaction. This avoids the workflow challenges of the enzymatic process, but typically results in lower yields.
Like ARCA, CleanCap® is a synthetic, chemically made mRNA 5’ cap analog added to the transcription process in a single step. Unlike ARCA, however, CleanCap® results in significantly higher levels of capping efficiency, resulting in very low levels of uncapped mRNA, which in turn minimizes the risk of activation of the innate immune system. In addition, CleanCap®’s higher mRNA yields compared to ARCA result in lower cost of goods. When compared to enzymatic capping, CleanCap® removes the additional downstream purification steps
139
required. We have developed a suite of CleanCap® analogs that are specifically designed for therapeutics and vaccines. CleanCap® products represented 54% of our nucleic acid production revenue for the nine months ended September 30, 2020.
mRNA. mRNA is an intermediary molecule that translates the genetic information stored in DNA into proteins. The genetic information stored in DNA is transferred to mRNA in a cellular process called transcription. This process occurs in the nucleus of cells. DNA, a double stranded molecule, is unwound and copied as mRNA by the enzyme RNA polymerase. mRNA is then transferred out of the nucleus to the cytosol, a component of the cytoplasm of a cell, where it serves as a blueprint for making cellular proteins by a multi-component organelle complex called the ribosome.
mRNA has traditionally been a difficult molecule for vaccine and therapeutic purposes. mRNA is inherently unstable compared to DNA and is susceptible to degradation by ubiquitous enzymes called RNases. mRNAs are also physically and chemically fragile and can degrade at elevated temperatures and under shear forces that occur during downstream manufacturing processes. We have developed manufacturing processes that overcome some of these obstacles, resulting in highly effective mRNA.
We develop and manufacture mRNA products to support vaccine and therapeutic programs from pre-clinical development through and including clinical phases, including scale-up and analytical development services. The mRNA molecules may serve as APIs for diverse applications, such as enzyme replacement therapies, gene editing therapies and vaccines. We offer both research grade material and material made under GMP conditions for early phase clinical trials. mRNA products represented 56% of our nucleic acid production revenue for the nine months ended September 30, 2020 (including the revenue from CleanCap® products).
Oligonucleotides. The oligonucleotide product category supports broad customer applications, including therapeutics, in vitro diagnostics, next generation sequencing (“NGS”) and CRISPR-based gene editing. Most of our TriLink BioTechnologies oligonucleotide products are custom manufactured DNA or RNA sequences, often highly modified and produced as research grade or under GMP conditions for use in development, clinical and commercial applications.
We also provide nucleoside triphosphates (“NTPs”). NTPs are the precursors to DNA and RNA. They are composed of a nitrogen base bound to either ribose or deoxyribose with three phosphate groups added to the sugar. We manufacture NTPs that are used in polymerase chain reactions (“PCR”), sequencing reactions and in the manufacture of mRNA. The NTPs can be unmodified, composed of the four standard bases, or modified, with a base altered to enhance a particular biological property, such as the ability to evade the innate immune system in therapeutic applications. TriLink BioTechnologies NTPs are used by customers in both research and clinical trial applications.
Our product offerings also include reagents that form the building blocks of oligonucleotides with our Glen Research products, including high quality specialty chemicals and amidites. The oligonucleotide products category represented 44% of our nucleic acid production revenue for the nine months ended September 30, 2020.
Plasmid DNA. We finished construction of a plasmid DNA manufacturing suite inside our newly built San Diego facility and manufacturing is expected to begin in the first quarter of 2021. Unlike genomic DNA, which constitutes the chromosome, plasmid DNA exists outside the chromosome and represents small circular double-stranded constructs. Plasmid DNA is frequently used as a vector for replicating nucleic acid products. Plasmid DNA is integral to the production of mRNA and our production of plasmid DNA will assist in ensuring the quality of the mRNA we produce.
Our nucleic acid customers are generally vaccine and therapeutic drug makers, who accounted for 60% of nucleic acid production revenue for the nine months ended September 30, 2020 and in vitro diagnostics and next-generation sequencing products manufacturers, who accounted for 23% of our nucleic acid production revenue over the same period.
140
Biologics Safety Testing (22% of Revenue for the Nine Months Ended September 30, 2020)
We provide products and services under the Cygnus Technologies brand that ensure the purity of our customers’ biopharmaceutical products, including biological drugs. For over 20 years, the Cygnus Technologies brand has been associated with products and services that enable the detection of impurities and contaminants present in bioproduction. Our biologics safety testing products are used during development and scale-up, during the regulatory approval process and throughout commercialization. We are recognized globally for the detection of HCPs and process-related impurities during bioproduction.
Our customers in this segment manufacture a broad range of biopharmaceutical products. These include monoclonal antibodies and recombinant proteins, both as novel biologics and biosimilars, and recombinant vaccines including vaccines to prevent COVID-19 and to treat cancer. We also provide products in support of the development of cell and gene therapies. Recombinant vaccines and cell and gene therapies rely on manufacturing of various viral vectors produced using recombinant nucleic acid and cell culture technologies. Viral vector manufacturing processes require rigorous analytics, including testing for process-related impurities such as HCPs, host cell DNA, purification leachates, growth media additives and enzymes used in viral vector purification processes.
ELISA is the benchmark method for monitoring levels of process-related impurities during the purification process and in product release testing. The advantages of well-developed ELISA kits include the ability to measure very low levels of impurities in the presence of high amounts of drug product, without requiring a high level of expertise to run, and are readily transferable across an organization from process development to manufacturing and quality control bioanalytical groups. Though relatively simple to run, these ELISA kits require a high level of expertise to design, develop and qualify.
Customers establishing biopharmaceutical manufacturing processes may use off-the-shelf or generic HCP kits provided by manufacturers like ourselves, or they may choose to design their own in-house assays for their specific processes. Some customers may choose to use generic assays early in development and migrate to process-specific assays later. The trend in recent years has been for customers to increasingly use generic assays throughout their development pathway, relying on our expertise and the established performance of our assays. If customers choose to develop their own assays, we offer custom services and bulk materials to assist them.
Our comprehensive catalog of Cygnus Technologies HCP ELISA kits covers 22 expression platforms and provides the specificity and sensitivity to detect impurities with reproducibility, which supports regulatory compliance. Our reputation for quality is recognized by the industry and global regulatory agencies, with Cygnus Technologies assays used as reference methods throughout the industry and to support manufacturing and quality control of commercialized biologics.
Our customers in this segment are biopharmaceutical companies, contract research organizations (“CROs”), contract development and manufacturing organizations (“CDMOs”) and life science companies, which together accounted for 46% of our biologics safety testing revenue for the nine months ended September 30, 2020. International distributors and United States-based resellers accounted for 53% of this revenue. These customers largely serve the biopharmaceutical industry. Academia, hospitals and government accounts contributed 1% of our biologics safety testing revenue in the nine months ended September 30, 2020.
141
Cygnus Technologies product categories include HCP ELISA kits, other bioprocess impurity and contaminant ELISA kits, ancillary reagents and custom services.
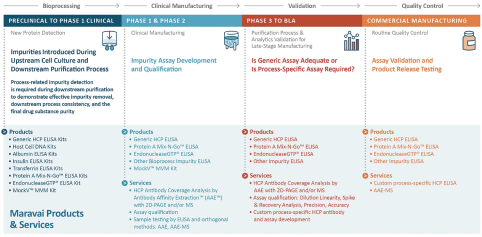
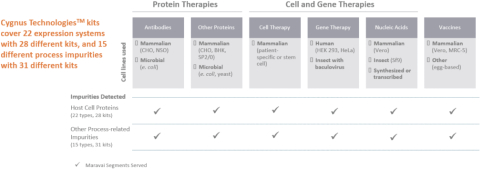
HCP ELISA kits. HCP ELISAs are kits used to detect residual proteins from the expression system used in bioproduction. HCPs constitute a major group of process-related impurities produced using cell culture technology no matter what cell expression platform is used. HCPs pose potential health risks for patients and the risk of failure of safety endpoints for drug manufacturers. When present in the administered product, even at low levels, HCPs can induce an undesired immune response, interfere with drug efficacy and impact drug stability. HCPs are a critical quality attribute for biologics safety testing development and must be adequately removed during the downstream purification process.
HCP ELISA kits represented 62% of our biologics safety testing revenue in the nine months ended September 30, 2020.
Other impurity and contaminant kits. Products in this category include kits for measuring Protein A leachate, which results from the affinity purification method used for monoclonal antibody therapeutic agents; ELISA kits for measuring additives in growth media, such as bovine serum albumin; ELISA kits for measuring host cell DNA; and ELISA kits to detect and quantify residual endonuclease impurities in recombinant viral vector and vaccine preparations.
In addition, in 2020, Cygnus Technologies introduced the MockV™ Minute Virus of Mice (MVM) kit, a novel, proprietary viral clearance prediction tool that includes a non-infectious “mock virus particle” mimicking the physicochemical properties of live virus that may be present endogenously in the drug substance or introduced during bioproduction. The kit enables manufacturers to conduct viral clearance assessments easily and economically and to predict outcomes in-house ahead of costly and logistically challenging live viral clearance studies.
142
Other impurity and contaminant kits represented 19% of our biologics safety testing revenue for the nine months ended September 30, 2020 in the biologics safety testing segment.
Ancillary reagents. These products include antibodies, antigens, sample diluents and other auxiliary products necessary to optimize applications for customer processes. Ancillary reagents represented 11% of our biologics safety testing revenue for the nine months ended September 30, 2020.
Custom services. We provide process-specific antibody and ELISA development, qualification and maintenance services. In addition, we have pioneered advanced orthogonal methods including antibody affinity extraction (AAETM) and mass spectrometry for HCP antibodies coverage analysis and HCP identification, which we provide as custom services. Custom services represented 8% of our biologics safety testing revenue for the nine months ended September 30, 2020.
Protein Detection (9% of Revenue for the Nine Months Ended September 30, 2020)
We believe that we are a leader in labeling and detection reagents for immunohistochemistry, immunofluorescence and glycobiology, principally in research settings, with Vector Laboratories, the brand under which we market our protein detection products, having been cited over 350,000 times in scientific publications. Our products are used to detect the expression of proteins in tissue, which may indicate an ongoing disease process, with the use of antibody-based detection systems. We also manufacture lectins, proteins that preferentially bind to carbohydrates and which are used, for example, in the study of glycosylation, the process by which carbohydrates attach to proteins and lipids. Glycosylation is critical in a range of biological processes, including cell-to-cell adhesion, the performance of glycoprotein-based drugs and cancer. In addition, we manufacture bioconjugation reagents to allow rapid and quantifiable conjugation of all classes of biomolecules.
Our presence in protein detection dates to the founding of Vector Laboratories in 1976. Under the Vector Laboratories brand, we provide reagents to researchers worldwide investigating biological processes and the nature of disease in tissue, including oncology research applications. Our reagents span the immunohistochemistry and immunofluorescence workflows and include products for tissue preparation, tagging targets of interest via secondary antibodies, detection systems for visualizing proteins, enzyme substrates for chromogenic color development, secondary antibodies to amplify target signal, fluorescent dyes for use in live cell imaging, fluorophore-conjugated secondary antibodies and products for the identification and isolation of glycosylated targets.
Our expertise includes the development of a broad range of highly validated secondary antibodies, used for labeling targets of interest. We produce over 20 proprietary antibodies constituting over 180 different SKUs in different formats and quantities. We also offer a broad portfolio of over 35 distinct lectins making up nearly 140 SKUs addressing a broad set of applications. Our capabilities extend to assay development, protein purification and bioconjugation as well as development of critical related reagents such as mounting media and substrates.
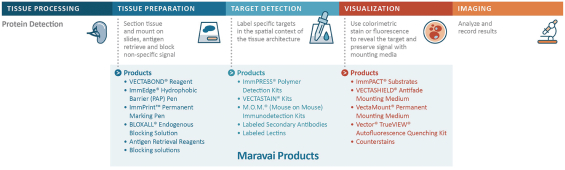
143
We principally serve academic researchers worldwide in our protein detection segment. Our research customers generally rely on us to provide our catalog products in a timely fashion, often overnight, and to provide live technical support and responsive customer service. All protein detection products sold directly to academic researchers carry the Vector Laboratories brand. We also sell custom products to industry customers, whether as components to be integrated into their own products, or to be resold. We serve these customers with catalog products directly via Web, email and phone ordering; with custom or bulk products through direct sales; and through distributors and resellers. Direct catalog sales represented 26% of our protein detection revenue for the nine months ended September 30, 2020. Bulk and custom products sold directly accounted for 30% of our protein detection revenue and resellers and distributors together accounted for 44% of our protein detection revenue during the same period.
Our Competitive Strengths
We believe we are a leader in providing nucleic acid products and biologics safety testing products and services to biopharmaceutical customers worldwide. Our success is built on the ability of our proprietary technologies and products, provided under exacting quality standards, to reliably serve our customers’ needs for critical raw materials.
Leading Supplier of Critical Solutions for Life Sciences from Discovery to Commercialization
We seek to be an important component of our customers’ supply chain by providing inputs that are central to the performance of their products and processes throughout the product lifecycle. By collaborating with customers early in the development phase, our products frequently follow our customers’ development path to commercialization and are likely to be incorporated as raw materials in their on-market products and processes. Our decades-long experience and track record, coupled with our ongoing investment in facilities and quality systems, allow our customers to rely on us for their critical products. Our approach is to be a trusted partner throughout the life cycle of our customers’ products.
Innovation, Proprietary Technologies and Knowhow Underpin Our Portfolio
Our expertise in complex chemistries leads customers to seek our collaboration in designing complex products that meet high performance expectations. Based on the responses to the Industry Analysis, we believe the solutions we provide, in many cases, cannot be provided effectively by our competitors. In certain cases, like our CleanCap® technology, our knowhow is backed by intellectual property. In other cases, such as our HCP products, our antibodies are proprietary and therefore can only be supplied by us. We believe the proprietary nature of our knowhow and products solidifies our long-term customer relationships.
Products with Outstanding Quality Performance
We believe our products stand out when compared to our competitors’ because they present innovative solutions to customer needs, as indicated by the responses to the Industry Analysis, while providing reliable performance and quality. CleanCap®, for example, offers advantages over competing technologies in yield, stability and safety. Our oligonucleotides address complex chemistry challenges, which we believe few competitors can address. The results of the Industry Analysis indicate that our HCP ELISAs have defined the market for impurity detection and we believe they have become a de facto standard in biologics safety testing. Similarly, our protein detection assays have been recognized for their performance for over 40 years.
Trusted Brands
Our TriLink BioTechnologies, Glen Research, Cygnus Technologies and Vector Laboratories brands are well known in their respective markets for consistent quality and performance. This brand recognition has been earned over decades. Our manufacturing processes, quality standards, technical support and high-touch customer service ensure that we maintain the reputation of our brands.
144
State-of-the-Art Manufacturing Facilities
Our biopharmaceutical customers manufacture their products to meet stringent quality standards and expect their critical suppliers to meet their exacting requirements. Our customers further expect that we have the production capacity to meet their needs in a timely manner. As of September 30, 2020, we estimated that $70 million has been invested in our flagship San Diego facility and its five dedicated manufacturing suites to produce materials under GMP conditions, along with the required quality systems to meet requirements specified by our customers. We similarly invest in our other sites to ensure we meet our customers’ expectations. We believe that the capacity to manufacture to stringent biopharmaceutical standards is constrained in the industry and our ability to meet this demand sets us apart from our competition.
Experienced Leaders and Talented Workforce
Our management includes experienced leaders with demonstrated records of success at Maravai and other highly regarded industry participants. In addition, as of September 30, 2020, approximately 17% of our workforce have earned advanced degrees and all receive rigorous on the job training. We believe the quality of our personnel is critical to ensuring the collaborative, long-standing relationships we maintain with many of our customers.
Our Markets
We participate in three distinct market segments: nucleic acid production, biologics safety testing and protein detection, which together represented approximately $8.4 billion in annual spending in 2019 and which are expected to grow at a 15% compound annual growth rate (“CAGR”) through 2023. Of that combined market, we estimate our addressable portion represents approximately $3.6 billion. Our addressable segments as a whole, adjusted for the mix of products we offer, are expected to grow at a weighted average blended rate of 20% per annum through 2023, according to industry consultants and management estimates. We benefit from favorable industry dynamics in our broader market segments and specific growth drivers in our addressable market segments.
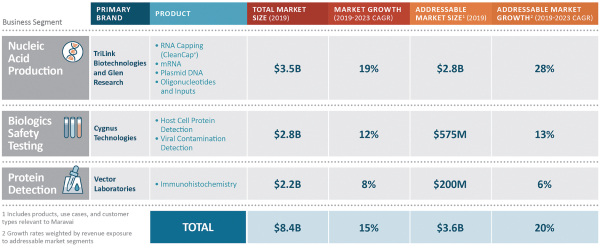
Biopharmaceutical customers are increasingly relying on outside parties to provide important inputs and services for their clinical research and manufacturing, a development driving growth for suppliers with unique capabilities and the ability to manufacture at an appropriate scale to support customer programs. We believe that suppliers like ourselves, with this rare combination of capabilities, proprietary products and the required investment in manufacturing and quality systems, are benefiting from rapid growth as biopharmaceutical customers seek to partner with a small number of trusted suppliers.
145
In addition to the continued trend toward outsourcing, several market developments are driving increased growth, above the broader market growth rates, in our addressable market segments, including:
| • | Pivot toward mRNA vaccines driven in part by COVID-19. mRNA vaccine pre-clinical programs grew approximately 38% in 2019, before the COVID-19 pandemic. That rate is estimated to increase to approximately 63% in 2020. The increased growth is being driven, in part, by 26 COVID vaccine programs using mRNA as of November 3, 2020, according to the World Health Organization. Five of the 26, including one phase II/III clinical program led by Pfizer in partnership with BioNTech, three phase I/II clinical programs led by Imperial College London, Fosun Pharma in partnership with BioNTech and CureVac and one pre-clinical program led by the University of Tokyo in partnership with Daiichi-Sankyo, involve our CleanCap® products and up to three more in earlier stages of development, led by Chula Vaccine Research Center in partnership with the University of Pennsylvania, eTheRNA Immunotherapies and Greenlight Biosciences, are currently using our CleanCap® products. Given the early stage of these three programs, there can be no assurance they will continue to use CleanCap® through commercialization. The mRNA vaccine technology is gaining prominence as a result of its faster development time, lower manufacturing costs and improved safety because of the lower risk of unwanted immune responses. RNA expertise is highly specialized and customers seek partners to provide these complex products. A small number of providers, like ourselves, can provide this RNA capability. |
| • | Rapid growth in development of cell and gene therapies. Sales of cell and gene therapy drugs are expected to grow from $1 billion in 2019 to $25 billion by 2024 and recent approvals of Kymriah®, Yescarta® and Luxturna™ have added clinical credibility to cell and gene therapies. We support the development of cell and gene therapies with products used in gene editing and cell therapy research, and we are well positioned to supply materials for gene therapy with our launch of DNA plasmid products, which we expect in the first quarter of 2021. |
|
|

|
146
| • | Large and growing pipeline of protein-based therapeutics. In addition to cell and gene therapies, an increase in protein-based therapies is driving the need for impurity testing during process development and manufacturing. |
| Protein Therapy Pipeline Growth CAGR = 9%
|
Protein Therapy Pipeline Mix CAGR = 9%
|
| Source: | Industry consultants. Included proteins are biosimilars, antibodies, recombinant proteins, hormonal products and coagulation factors |
| • | Rise in molecular diagnostics driven by COVID-19. The market for molecular diagnostics is growing dramatically because of demand for new tests related to COVID-19. This growth is driving demand for our products, particularly oligonucleotides and related inputs. |
| • | COVID-19 providing both short-term and expected long-term growth. Several of our product categories are experiencing accelerated growth in 2020, notably our CleanCap® and oligonucleotide products. We expect the impact of COVID-19 on our growth to sustain in the longer-term as the entire mRNA category benefits from lessons learned during the COVID-19 pandemic. We expect research in other therapeutic categories to experience increased growth as research conducted for COVID-19 diffuses more broadly into other vaccines and therapies. |
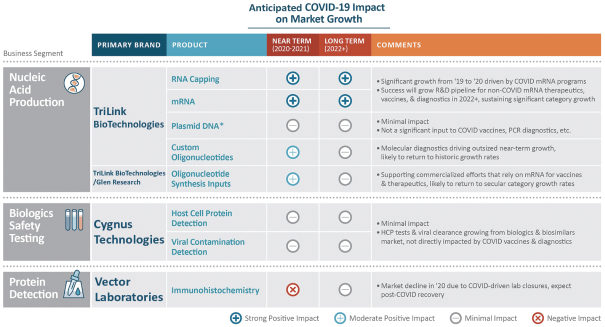
| * | Our plasmid DNA products are expected to launch in Q1 2021. |
147
Nucleic Acid Production Market
The nucleic acid production market includes the production and synthesis of reagents for research and manufacturing of DNA and RNA-based biologics. Nucleic acid production is a $3.5 billion market expected to grow at 19% annually through 2023. Growth has generally accelerated in recent years with continued innovation in cell and gene therapy, including mRNA therapeutics and synthetic biology approaches. Our addressable portion of the market is $2.8 billion with expected growth of 28% annually through 2023. This higher growth rate is driven by our exposure to high growth sub-markets including RNA cap analog production and mRNA. Capping and mRNA growth is fueled by the continued growth of nucleic acid vaccines and therapies, which we expect will accelerate because of research into COVID-19. That research has highlighted the benefits of mRNA vaccines and therapies more broadly.
The field of mRNA-based drugs and vaccines has advanced dramatically within a few short years. Capacity to manufacture these products when approved, however, remains in short supply. Providers of technical expertise and manufacturing capabilities, like ourselves, with the facilities and quality systems demanded by biopharmaceutical customers, benefit from the demand created in the mRNA category.
COVID-19 is further accelerating growth in custom oligonucleotides and related inputs, which are used to manufacture diagnostic tests. New participants have entered the diagnostics market. Reference labs and hospitals have rapidly expanded their capacity. And demand for testing is increasing rapidly. These developments in turn lead to increased demand for our oligonucleotide products.
Biologics Safety Testing Market
The biologics safety testing market includes the detection and clearance of downstream bioprocessing product and process impurities. Biologics safety testing is a $2.8 billion market expected to grow at 12% annually through 2023. We participate in the HCP and other process related impurities and viral contamination segments of this market for biopharmaceutical vaccine and therapeutics manufacturing. These addressable segments account for $575 million of the market and are expected to grow at 13% annually through 2023. The growth in this market is driven by continued growth of biologics and biosimilars and increased outsourcing of process development.
Protein Detection Market
The protein detection market includes methods to detect and visualize proteins (antigens) in tissue sections to provide insight into gene expression, spatial relationships, and biomarker identification. Protein detection includes immunohistochemistry, immunofluorescence and glycobiology. Immunohistochemistry, our largest market within protein detection, is a $2.2 billion market, expected to grow at 8% annually through 2023. We participate in the immunohistochemistry segment of the academic and biopharmaceutical research market, which represents $200 million of annual expenditures expected to grow at 6% annually through 2023. This market has seen a temporary contraction in 2020 of approximately 15% given lab closures due to COVID-19, but is expected to return to historical numbers.
Our Strategy
Our customers strive to improve human health. Our goal is to provide them with products and services to accelerate their development efforts, from basic research through clinical trials and ultimately to commercialization for drugs, diagnostics and vaccines.
Supporting Biopharmaceutical Customers from Product Development Through Commercialization
Our customers include both emerging and established biopharmaceutical leaders developing novel therapies, diagnostics and vaccines. Emerging biopharmaceutical customers frequently seek the support we can offer in our
148
state-of-the-art facilities under our stringent quality standards, with the capabilities that result from the capital and process investments we have made over the last several years. We are capable of manufacturing reagents from research-grade to GMP-grade, which often exceeds the in-house capabilities of our pre-commercial customers. The results of the Industry Analysis indicate that our emerging and established customers also seek us out for our leading capabilities in nucleic acid chemistries and process control assays. We have expertise in complex chemistries, especially in highly modified nucleic acids and mRNA, and we believe we are a leader in applying these capabilities to the development of vaccines and therapeutics. We further support our customers as they transition from product development to commercialization by providing critical raw materials for their drugs. A core component of our strategy is the continued investment in facilities, quality standards and products and services that allow us to support our customers through the entire life cycle of their drugs.
Developing Proprietary Technologies that Deepen our Relationships with Our Customers
We are experts in nucleic acids and our scientists aim to develop proprietary enabling technologies that become integral to our customers’ products. For example, CleanCap®, our proprietary chemical capping technology, has demonstrated its advantages in terms of the stability of the associated mRNA and its efficiency in protein production when compared to traditional capping technologies. This efficiency has led biopharmaceutical customers to employ CleanCap® in their vaccine and therapeutic programs. As those products proceed through development into commercialization, we believe CleanCap® will be a critical input in on-market vaccines and therapeutics, with over 100 customers having used CleanCap® as of September 30, 2020 and five COVID-19 vaccine programs incorporating CleanCap® as of September 30, 2020, including one phase II/III clinical program led by Pfizer in partnership with BioNTech, three phase I/II clinical programs led by Imperial College London, Fosun Pharma in partnership with BioNTech and CureVac and one pre-clinical program led by the University of Tokyo in partnership with Daiichi-Sankyo. We expect to supply our customers throughout their products’ life cycle.
Forming Long-Term Partnerships for Critical Biopharmaceutical Components and Process Tests
Our products are frequently incorporated into regulated and highly validated therapeutic and diagnostic products and processes. Our biopharmaceutical customers expect us to provide them with consistent, high quality products that meet narrow specifications, and that we ensure their supply chain for such products for the length of their programs. In many cases, we may be the sole source of the products we provide. We therefore take seriously our responsibility to our biopharmaceutical partners, and by extension the patients they serve. Our emphasis on partnership generally leads to long-term relationships with our customers.
Focusing Our Efforts on High Growth End Markets
While biopharmaceutical research and in vitro diagnostics markets are experiencing strong growth, we target the highest growth segments within those markets. Our product portfolio is well positioned to serve the biologic, cell and gene therapy and mRNA vaccine and therapeutic end markets, which are currently experiencing above-market growth. By investing in technologies at the forefront of biopharmaceutical and in vitro diagnostics, we aim to remain focused on the highest-growth applications.
Opportunistically Acquiring Leading Life Sciences Businesses and Supporting Their Continued Development
We built our business by acquiring established and emerging companies with strong scientific foundations in our target markets and investing in their systems, processes and people to accelerate their growth and expand their technologies. Going forward, we may opportunistically pursue strategic acquisitions that we believe meet, or could meet after being acquired and expanded, the following criteria:
| • | address our core target markets; |
| • | have a demonstrated adherence to high quality standards; |
149
| • | be leaders in their market niches; |
| • | have differentiated or proprietary products and processes that provide clear value to our biopharmaceutical and other customers; and |
| • | have a track record of attractive rates of growth and compelling returns on invested capital. |
Our acquisition strategy is to invest significantly in our acquired businesses. We strive to rapidly integrate their information and financial systems. All of our companies share a common enterprise resource planning system and we implement our financial controls and reporting systems soon after acquisition. We seek opportunities to invest in their facilities and personnel to provide an operating foundation for growth. We also augment their commercial capabilities through a combination of sales and marketing resources dedicated to each business, supported by our global marketing infrastructure.
We will continue to seek a balance between driving growth organically and through opportunistic acquisitions.
Commercial
We have relationships with the following categories of customers (percentages represent the share of revenue in each category for the nine months ended September 30, 2020): developers of therapeutics and vaccines (63%), other biopharmaceutical and life science research companies (29%), academic institutions (5%) and molecular diagnostic companies (3%). Our biopharmaceutical customers include startups, established biotechnology companies and large pharmaceutical companies developing enzyme replacement therapies, gene editing therapies, ex vivo therapies and vaccines.
Our commercial function includes direct sales, marketing, customer service, technical support and distributor management. We serve customers through direct sales in each business segment, with a primary focus on our larger biopharmaceutical and other industry customers. We serve our academic customers via Web, email and phone ordering. We support all customers with live technical support and customer service.
We address customers outside the United States with a combination of direct sales and distributors. We serve many of our biopharmaceutical customers, especially in our nucleic acid production segment, via direct sales worldwide. Our distributors also sell our products in over 50 countries and provide customer service and local sales and marketing. As of September 30, 2020, our commercial organization included 56 employees and over 100 distributors.
Competition
We compete with a range of companies across our segments.
Nucleic Acid Production
Within nucleic acid production, we compete with four primary types of companies: (1) chemistry companies that create and produce the basic monomers, amidites, and supports that go into the creation of an oligonucleotide; (2) oligonucleotide manufacturers that specialize in custom oligonucleotide development of varying complexities and scales; (3) mRNA biotechnology companies that create fully processed mRNA and specialize in custom, complex orders; and (4) CDMO organizations that have the capability to accept work from large biopharmaceutical companies and serve as the outsourcing entity for the development and manufacturing of nucleic acid products. However, it is important to note that CDMOs seldom offer proprietary products.
For mRNA capping analogs, we compete principally with Thermo Fisher Scientific Inc. (“Thermo Fisher”) and Hongene Biotech Corporation, who offer alternatives to CleanCap®. Many biopharmaceutical companies produce capping solutions in-house using enzymatic or ARCA processes. However, given CleanCap®’s high
150
yield and process efficiency, many customers who previously insourced these processes have begun to partner with us. Based on the Industry Analysis, we believe our products and services are more effective than those of our competitors. Deep scientific expertise, intellectual property protection and specialty equipment serve as barriers to entry in this space.
For our mRNA offerings, we compete with Aldevron LLC, Bio-synthesis Inc., and System Biosciences, LLC, among others. Based on the Industry Analysis, we believe we have a reputation for our expertise in the RNA space with talented scientists who are constantly pushing the frontier of RNA science. This scientific expertise and the required high-cost equipment serve as barriers to entry. In addition to our expertise, we believe our GMP cleanroom manufacturing process differentiates us from competitors.
For custom oligonucleotides, we compete with a number of manufacturers. Custom oligonucleotide providers include those that provide complex, highly modified oligonucleotides and those that provide less complex offerings. In the custom oligonucleotide space, complexity is based on the length of the sequence and level of modification to the phosphate backbone. Large manufacturers like Integrated DNA Technologies, Inc., Thermo Fisher and EMD Millipore Corporation (“Millipore Sigma”) serve less complex customer needs while TriLink BioTechnologies, LGC Biosearch Technologies, Inc. and GenScript Biotech Corporation serve more complex customer needs. In the custom oligonucleotide market, we have a reputation for accepting complex orders and delivering high purity products that reduce researcher re-work and save money. Quick turnaround times and the ability to produce at scale are essential requirements in this segment.
In the oligonucleotide synthesis inputs market, we compete against large distributor-manufacturers like Thermo Fisher and Millipore Sigma while also serving them as customers. The Glen Research brand has a long history in this industry, which drives customer loyalty, and has a reputation for high-fidelity technical service, focusing on supplying and sourcing highly modified inputs for its customers.
Biologics Safety Testing
For drugs in early development, we compete against other bioprocess impurity kit providers such as BioGenes GmbH (“BioGenes”) or Enzo Life Sciences, Inc. (“Enzo”). Competitors generally offer fewer expression platforms (as few as one or two) compared to our offering of 21 expression platforms and a total of 78 ELISA impurity detection kits. As a drug successfully moves forward to validation and approval stages, a customer may either continue with an off-the-shelf kit or they may begin the process to develop a custom assay that is tailored to meet their specific host cell and manufacturing process needs. During the entire drug development process, and especially during this decision, we are partners with the manufacturer and provide our expertise to help them make the best bioprocess quality control and testing-related decisions.
If a drug manufacturer continues with an off-the-shelf assay from development to validation and approval, they will generally stay with the incumbent kit provider due to the extensive validation they have conducted. For custom assay development, our main competitors are BioGenes, Rockland Immunochemicals, Inc., and some CDMOs and CROs with custom assay development capabilities. The trend in recent years has been for CDMOs, CROs and large biopharmaceutical companies to focus on core competencies and outsource host cell protein assays or qualify off-the-shelf kits when possible.
Protein Detection
In the protein detection market, we compete against large life sciences manufacturers and niche tissue staining offerings. We compete in the research segment of this market against large life sciences manufacturers such as Thermo Fisher and Abcam plc, who compete across the value chain offering primary and secondary antibody detection, visual detection and labeling, slide processes and visualization and analysis. Additionally, we compete against niche tissue staining offerings such as Enzo and Jackson ImmunoResearch Laboratories, Inc. We are differentiated by our deep visual detection and labeling experience, our Vector Laboratories brand’s
151
sterling reputation established over more than 40 years, and the desire of our research customers to replicate past findings, many of which were completed using Vector Laboratories products.
Licenses and Collaborations
ProteinSimple Supply and Distribution Agreement
On August 12, 2019, we (through Cygnus Technologies) entered into a Supply and Distribution Agreement with ProteinSimple (the “ProteinSimple Agreement”) for the supply of bioprocess impurity assays to be assembled in assay cartridges for use in automated analyzer instruments. Under the ProteinSimple Agreement, we supply to ProteinSimple, at no charge, certain reagents to be incorporated into ProteinSimple cartridges or sold directly by us. This collaboration with ProteinSimple is generally exclusive in the field of testing for bioprocess impurities using immunoassays on an automated analyzer for the United States, United Kingdom, Ireland and Europe.
The ProteinSimple Agreement contains non-exclusive licenses from each party to the other to permit the other party to fulfill its obligations and sell its products under the ProteinSimple Agreement. If any intellectual property is developed jointly or any intellectual property that covers both bioprocess impurity assays and automated immunoassay kits and instruments is developed solely by either party, we will own all intellectual property with respect to the bioprocess impurity assays and ProteinSimple will own all intellectual property with respect to automated immunoassay kits and instruments.
The ProteinSimple Agreement is in effect for an initial seven (7) year term with automatic renewal for successive two (2) year renewal terms unless either party elects not to renew. Beginning on the third anniversary of the date of the ProteinSimple Agreement, either Party may terminate the ProteinSimple Agreement on thirty (30) days’ notice if Cygnus Technologies has not purchased certain minimum numbers of cartridge kits from ProteinSimple.
Broad Patent License Agreement
We (through TriLink BioTechnologies) entered into a Nonexclusive Patent License and Material Transfer Agreement with The Broad Institute, Inc. (“Broad”) effective as of July 5, 2017, and amended on September 29, 2017 (the “Broad Patent License Agreement”). Broad, together with a consortium of educational institutions (including Harvard University and the Massachusetts Institute of Technology), owns and controls certain patent rights relating to genome editing technology, including the CRISPR-Cas9 gene editing processes and have a licensing program for use and commercialization of technologies and products covered by the underlying patent rights. Under the Broad Patent License Agreement, Broad grants to us a non-exclusive, royalty-bearing, non-transferable and non-sublicensable, worldwide license under the licensed patent rights to manufacture and sell products and to perform certain in vitro processes or services on a fee-for-service basis, in each case, solely as research tools for research purposes (excluding human, clinical or diagnostic uses). We must use diligent efforts to develop products, introduce products into the commercial market and make products reasonably available to the public. We are obligated to pay a mid-five figure annual license maintenance fee and royalties in the range of 5% to 10% on net sales of covered products and processes.
The term of the Broad Patent License Agreement extends through the expiration of the last to expire claim of any of the licensed patents. We are entitled to terminate the Broad Patent License Agreement for convenience at any time on at least three (3) months’ written notice, in which case we must continue to pay license maintenance fees and royalties as noted above for the sale of products that are not covered by the specific claims of the licensed patent rights but are otherwise derived from such licensed patent rights or from products covered by such licensed patent rights. Broad may terminate the license for our uncured failure to make payments, for our uncured material breach or if we bring a patent challenge against any of the institutional rights holders.
152
LSU Patent License Agreement
We (through TriLink BioTechnologies) entered into a Patent License Agreement with the Board of Supervisors of Louisiana State University and Agricultural and Mechanical College and Dr. Edward Darzynkiewicz (collectively, “LSU”) effective as of July 7, 2010 (the “LSU Patent License Agreement”). Under the LSU Patent License Agreement, LSU grants to us a non-exclusive, royalty-bearing license under an issued U.S. patent and patents that claim priority thereto, directed to mRNA capping technology to make and sell reagents and kits for research use only (excluding use in humans or for diagnostic or therapeutic purposes) in the United States. We are required to use commercially reasonable efforts to commercialize the licensed products throughout the life of the LSU Patent License Agreement. We are obligated to pay a low four-figure annual license maintenance fee and royalties in the range of 5% to 10% on net sales of licensed products.
We must pay royalties to LSU until the expiration of the last to expire licensed patents. We are entitled to terminate the LSU Patent License Agreement for convenience at any time on at least sixty (60) days’ written notice, subject to paying in full all amounts due up to the date of termination and cessation of any exercise of the licensed rights thereafter. LSU may terminate the license for our uncured failure to make payments or our uncured material breach.
AmberGen Agreement
We (through Glen Research) entered into an Agreement with AmberGen, Inc. (“AmberGen”), dated May 11, 2000 (the “AmberGen Agreement”) under which AmberGen has appointed us the exclusive distributor of AmberGen’s proprietary photocleavable product offered under the name PC Phosphoramidite on a worldwide basis. We are limited to selling the product for research use only and are required to use good faith efforts to discontinue distribution to buyers making use of the product than purposes other than laboratory research.
We are entitled under the AmberGen Agreement to purchase product from AmberGen at AmberGen’s cost to manufacture the product. On a monthly basis, we are required to remit to AmberGen 50% of the gross profits on product sales for which payments were received in the preceding month.
The AmberGen Agreement was initially in effect for a five-year term but is now in a series of automatic one-year renewal terms. Either party may terminate the AmberGen Agreement on six months’ written notice or immediately for material breach of the other party or, subject to a cure period, for certain bankruptcy-related events.
BTI Biosearch Dyes Agreement
We (through Glen Research) are a party to a Commercial Supply and License Agreement with Biosearch Technologies, Inc. (“BTI”), dated June 29, 2004, as amended on November 8, 2004 (the “BTI Biosearch Dyes Agreement”), under which BTI agrees to supply us with certain BTI dyes and we are granted a worldwide, non-exclusive license to sell certain BTI dyes and to use BTI’s product-related trademarks to do so. The BTI dyes can only be sold for the customer’s internal research and development use and inclusion in commercial kits or any commercial application is prohibited unless the customer has obtained a valid commercial license from BTI. The rights granted do not include sales to customers for use in human in vitro or clinical diagnosis. We are required to pay a per unit price for the licensed BTI products.
The BTI Biosearch Dyes Agreement was originally in effect for a term of two years and is now in a series of annual year-to-year renewals. Either party has the right to opt-out of such renewals upon 90 days’ notice prior to the next renewal. Either party can terminate the agreement for convenience at any time on six months’ written notice. Either party can terminate the agreement for the other party’s uncured material breach or insolvency.
Manufacturing and Supply
We occupy facilities in San Diego, California, Burlingame, California, Southport, North Carolina and Sterling, Virginia. Except for our Sterling facility, all our facilities are engaged in the manufacture of reagents.
153
Our San Diego facility, in particular, was designed and built by us in conjunction with the building owner to contain fully functional chemical and biological manufacturing operations from material receiving to product distribution and has its own loading dock, manufacturing gas delivery system, solvent delivery and waste system, ISO 8 and ISO 7 designated customer manufacturing suites and integrated building management systems for required site control. Our San Diego facility, fully completed in early 2020, doubled our previous overall nucleic acid production manufacturing capacity and quintupled our previous capacity to manufacture products under certain GMP conditions.
We continue to invest in our San Diego facility with recent expansions allowing for the manufacture of plasmid DNA and creation of ISO Class 8 and ISO Class 7 clean rooms providing for an expansion of the scale at which we can manufacture CleanCap® and NTPs, supported by a pilot plant for development of large scale manufacturing processes.
Our Southport and Burlingame operations are engaged in the manufacture and processing of antibody, ELISA kits and related reagents. The facilities incorporate laboratory, manufacturing, bottling, shipping and waste handling capabilities. Our Sterling facility was designed to perform quality control, aliquoting, packaging and shipping and houses the appropriate space and systems.
Our supply chain relies on a network of specialized suppliers and transportation companies. We regularly review our supply chain for supplier quality and risks related to concentration of supply and we take appropriate action to manage these potential risks.
Government Regulation
We provide products used for basic research or as raw materials used by biopharmaceutical customers for further processing, and active pharmaceutical ingredients used for preclinical and clinical studies. The quality of our products is critical to researchers looking to develop novel vaccines and therapies and for biopharmaceutical customers who use our products as raw materials or who are engaged in preclinical studies and clinical trials. Biopharmaceutical customers are subject to extensive regulations by the Food and Drug Administration (“FDA”) and similar regulatory authorities in other countries for conducting clinical trials and commercializing products for therapeutic, vaccine or diagnostic use. This regulatory scrutiny results in our customers imposing rigorous quality requirements on us as their supplier through supplier qualification processes and customer contracts.
Our nucleic acid and biologics safety testing segments produce materials used in research and biopharmaceutical production, clinical trial vaccines and vaccine support products. We produce materials in support of our customers’ manufacturing businesses and to fulfill their validation requirements, as applicable. These customer activities are subject to regulation and consequently require these businesses to be inspected by the FDA and other national regulatory agencies under their respective cGMP regulations. These regulations result in our customers imposing quality requirements on us for the manufacture of our products, and maintain records of our manufacturing, testing and control activities. In addition, the specific activities of some of our businesses require us to hold specialized licenses for the manufacture, distribution and/or marketing of particular products.
All of our sites are subject to licensing and regulation, as appropriate under federal, state and local laws relating to:
| • | the surface and air transportation of chemicals, biological reagents and hazardous materials; |
| • | the handling, use, storage and disposal of chemicals (including toxic substances), biological reagents and hazardous waste; |
| • | the procurement, handling, use, storage and disposal of biological products for research purposes; |
| • | the safety and health of employees and visitors to our facilities; and |
| • | protection of the environment and general public. |
154
Regulatory compliance programs at each of our businesses are managed by a dedicated group responsible for regulatory affairs and compliance, including the use of outside consultants. Our compliance programs are also managed by quality management systems, such as vendor supplier programs and training programs. Within each business, we have established Quality Management Systems (“QMS”) responsible for risk based internal audit programs to manage regulatory requirements and client quality expectations. Our QMS program ensures that management has proper oversight of regulatory compliance and quality assurance, inclusive of reviews of our system practices to ensure that appropriate quality controls are in place and that a robust audit strategy confirms requirements for compliance and quality assurance.
Research Products
Our products and operations may be subject to extensive and rigorous regulation by the FDA and other federal, state, or local authorities, as well as foreign regulatory authorities. The FDA regulates, among other things, the research, development, testing, manufacturing, clearance, approval, labeling, storage, recordkeeping, advertising, promotion, marketing, distribution, post-market monitoring and reporting, and import and export of pharmaceutical drugs. Certain of our products are currently marketed as research use only (“RUO”).
We believe that our products that are marketed as RUO products are exempt from compliance with GMP regulations under the FDCA. RUO products cannot make any claims related to safety, effectiveness or diagnostic utility and they cannot be intended for human clinical diagnostic use. In November 2013, the FDA issued a final guidance on products labeled RUO, which, among other things, reaffirmed that a company may not make any clinical or diagnostic claims about an RUO product. The FDA will also evaluate the totality of the circumstances to determine if the product is intended for diagnostic purposes. If the FDA were to determine, based on the totality of circumstances, that our products labeled and marketed for RUO are intended for diagnostic purposes, they would be considered medical products that will require clearance or approval prior to commercialization.
We do not make claims related to safety or effectiveness and they are not intended for diagnostic or clinical use. However, the quality of our products is critical to meeting customer needs, and we therefore voluntarily follow the quality standards outlined by the International Organization for Standardization for quality management systems (ISO 9001:2015) for the design, development, manufacture, and distribution of our products. Some biopharmaceutical customers desire extra requirements including quality parameters and product specifications, which are outlined in customer-specific quality agreements. These products are further processed and validated by customers for their applications. Customers qualify us as part of their quality system requirements, which can include a supplier questionnaire and on-site audits. Customers requalify us on a regular basis to ensure our quality system, processes and facilities continue to meet their needs and we are meeting requirements outlined in relevant customer agreements.
Active Pharmaceutical Ingredients (APIs) for Clinical Trials
We provide APIs to customers for use in preclinical studies through and including clinical trials. We hold a drug manufacturing license with the California Food and Drug Branch of the California Department of Public Health for manufacture of APIs for clinical use and are subject to inspection to maintain licensure. Manufacture of APIs for use in clinical trials is regulated under § 501(a)(2)(B) of the FDCA, but is not subject to the current GMP regulations in 21 CFR § 211 by operation of 21 CFR § 210. We follow the principles detailed in the International Council for Harmonisation (ICH) Q7, Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients (Section 19, APIs For Use in Clinical Trials) in order to comply with the applicable requirements of the FDCA, and the comparable GMP principles for Europe; European Community, Part II, Basic Requirements for Active Substances Used as Starting Materials (Section 19, APIs For Use in Clinical Trials). APIs are provided to customers under customer contracts that outline quality standards and product specifications. As products advance through the clinical phases, requirements become more stringent and we work with customers to define and agree on requirements and risks associated with their product.
155
Customers’ biopharmaceutical products early in their development have a high failure rate and often do not advance through the clinical stages to commercialization. Our customers are required to follow regulatory pathways that are not always known, which may cause additional unforeseen requirements placed on us as their contract manufacturer and delays in advancing to the next stage of product development. We also provide novel compounds for cell and gene therapy applications, which result in additional challenges for our customers attempting to obtain regulatory approval given that this field is relatively new and regulations are evolving. Customer clinical trials rely on approval from institutional review boards (IRBs) and patient and volunteer enrollment, which makes timelines unpredictable for advancing to the next stage in product development. Preclinical studies and clinical trials conducted by our customers are also expensive and data may be negative or inconclusive causing customers to abandon projects that were expected to continue. Regulatory requirements in both the United States and abroad are always evolving and compliance with future laws may require significant investment to ensure compliance.
Other Regulatory Requirements
Select agent and toxin. We have one product classified as a select agent and toxin. Pursuant to the Public Health Security and Bioterrorism Preparedness and Response Act of 2002, the United States Department of Health and Human Services (“HHS”) and the United States Department of Agriculture (“USDA”) have established regulatory requirements for the possession, use, and transfer of biological agents and toxins that have the potential to pose a severe threat to public health and safety, animal and plant health, and the safety of animal and plant products for their intended use. These requirements can be found at 42 CFR Part 73 (HHS), 7 CFR Part 33 1 (USDA-PPQ), and 9 CFR Part 121 (USDA-VS). The possession, use and transfer of the relevant biological agent and toxin in quantities greater than 1.0 gram is governed by the regulations of 42 CFR Part 73 (HHS), Possession, Use and Transfer of Select Agents and Toxins. Vector Laboratories is registered with the CDC for these activities, is subject to inspection by the CDC and maintains an approved biosecurity plan. The regulations include specific requirements for safety (e.g. handling), security (e.g. access control, inventory control) and emergency response (e.g. addressing spills during manufacture or broken containers).
Environmental laws and regulations. We believe that our operations comply in all material respects with applicable laws and regulations concerning environmental protection. There have been no material effects upon our earnings or competitive position resulting from compliance with applicable laws or regulations enacted or adopted relating to the protection of the environment. Our capital and operating expenditures for pollution control in 2019 were not material and are not expected to be material in 2020.
Intellectual Property
Our success depends in part on our ability to obtain and maintain intellectual property protection for our products and services, defend and enforce our intellectual property rights, preserve the confidentiality of our trade secrets, and operate without infringing, misappropriating or otherwise violating valid and enforceable intellectual property rights of others. We seek to protect the investments made into the development of our products and services by relying on a combination of patents, trademarks, copyrights, trade secrets, including know-how, and license agreements. We also seek to protect our proprietary products and services, in part, by requiring our employees, consultants, contractors and other third parties to execute confidentiality agreements and invention assignment agreements.
Patents. Our intellectual property strategy is focused on protecting through patents and other intellectual property rights our core products and services, including CleanCap®, and related instrumentation and applications. In addition, we protect our ongoing research and development into critical reagents for cell and gene therapy through patents and other intellectual property rights.
As of September 30, 2020, we solely owned 18 issued U.S. patents including two patents received in December 2019 for certain of our CleanCap® products, three pending U.S. non-provisional patent applications,
156
29 issued foreign patents and 10 pending foreign patent applications and co-own two issued U.S. patents and one issued foreign patent with third parties, the details of which are set out in the tables below. Two of the foregoing U.S. patent applications are national stage filings of two PCT patent applications that we solely own. Our patent portfolio generally includes patents and patent applications relating to compositions and methods for the production of oligonucleotides, nucleic acids and mock viral particles. Issued U.S. patents in our portfolio of company-owned patents are expected to expire between 2021 and 2035. In addition, certain patents related to our SoluLINK products expired in 2020 and certain other patents related to such products are due to expire in 2021 and 2022.
The following patents and patent applications (including expected 20 year expiration dates) relate to our CleanCap® related products and technology.
| Patent and Patent Application Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 10,494,399, US 10,519,189, US Patent App. No. 15/761,957, and foreign applications in certain jurisdictions claiming priority to PCT/US2016/052670 | Owned | Sept. 20, 2036 | Directed to compositions and methods for synthesizing 5’-capped RNAs |
The following patents and patent applications (including expected 20 year expiration dates and any patent term adjustment) relate to our CleanTag® Library Prep related products and technology.
| Patent Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 8,728,725 | Owned | Jan. 5, 2032 | Directed to compositions and methods for ligation of nucleic acids | |||
| US 9,631,227 | Owned | Nov. 10, 2030 | Directed to compositions and methods for ligation of nucleic acids | |||
| AU Patent No. 2010270715 | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids | |||
| CA Patent No. 2,767,408 | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids | |||
| EP Patent No. 2451980 (validated in DE, ES, FR, GB, IT) | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids | |||
| ES Patent No. 2521740 | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids | |||
| HK Patent No. 1220234 | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids | |||
| JP Patent No. 5903379 | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids | |||
| MX Patent No. 321180 | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids |
157
| NZ Patent No. 597535 | Owned | July 6, 2030 | Directed to compositions and methods for ligation of nucleic acids |
The following patents and patent applications (including expected 20 year expiration dates and any patent term adjustment) relate to our CleanAmp® related products and technology.
| Patent Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 8,133,669 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| AU Patent No. 2009257815 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| CA Patent No. 2,725,239 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| CN Patent No. 102105481 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| EP Patent No. 2294076 (validated in DE, ES, FR, GB, IT) | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| ES Patent No. 2625938 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| GB Patent No. 2473778 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| HK Patent No. 1155456 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| IN Patent No. 318293 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| JP Patent No. 5712125 | Owned | May 21, 2029 | Directed to compositions and methods for nucleic acid replication | |||
| US 8,361,753 | Owned | October 21, 2029 | Directed to compositions and methods for nucleic acid amplification | |||
| AU Patent No. 2007268075 | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification | |||
| CA Patent No. 2,653,841 | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification | |||
| CN Patent No. 101517091 | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification | |||
| DE Patent No. 602007013223 | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification |
158
| EP Patent No. 2032714 (validated in DE, ES, FR, GB, IT) | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification | |||
| ES Patent No. 2360738 | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification | |||
| HK Patent No. 1129045 | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification | |||
| JP Patent No. 5558811 | Owned | May 17, 2027 | Directed to compositions and methods for nucleic acid amplification |
The following patent (including expected 20 year expiration dates and any patent term adjustment) relates to our decanoic acid diester linker-related technology.
| Patent Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 6,320,041 | Owned | April 13, 2021 | Directed to compositions used for chemical joining molecules to oligonucleotide |
The following patents and patent applications (including expected 20 year expiration dates and any patent term adjustment) relate to our SoluLINK® related products and technology.
| Patent Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 6,686,461 |
Owned | Feb. 28, 2021 | Directed to methods and compositions for preparation, detection and immobilization of macromolecules including oligonucleotides | |||
| US 7,173,125 |
Owned | Jan. 29, 2022 | Directed to methods and compositions for preparation, detection and immobilization of macromolecules including oligonucleotides | |||
| US 7,999,098 |
Owned | Jan. 29, 2022 | Directed to methods and compositions for preparation, detection and immobilization of macromolecules including oligonucleotides | |||
| US 6,800,728 |
Owned | June 28, 2021 | Directed to methods and compositions for crosslinking and immobilizing biomolecules, drugs and synthetic polymers |
159
| US 7,462,689 |
Owned | July 15, 2021 | Directed to methods and compositions for crosslinking and immobilizing biomolecules, drugs and synthetic polymers | |||
| US 7,732,628 |
Owned | Sept. 6, 2023 | Directed to methods and compositions for immobilizing biomolecules, drugs and synthetic polymers | |||
| US 7,102,024 |
Owned | Sept. 6, 2023 | Directed to methods and compositions for immobilizing biomolecules | |||
| US 6,911,535 |
Owned | Mar. 31, 2022 | Directed to methods for immobilizing biomolecules | |||
| US 8,541,555 |
Owned | April 8, 2031 | Directed to methods and compounds used to label biomolecules | |||
| US 8,846,875 |
Owned | Feb. 11, 2031 | Directed to methods, systems, and kits for preparing, purifying, and isolating oligonucleotide conjugates | |||
| EP Patent No. 1315699 (validated in FR, DE, GB) | Owned | Mar. 22, 2021 | Directed to methods and compositions for crosslinking and immobilizing biomolecules, drugs and synthetic polymers | |||
| EP Patent No. 2295407 (validated in FR, DE, GB) | Owned | Mar. 22, 2021 | Directed to methods and compositions for crosslinking and immobilizing biomolecules, drugs and synthetic polymers | |||
| EP Patent No. 2298736 (validated in FR, DE, GB) | Owned | Mar. 22, 2021 | Directed to methods and compositions for crosslinking and immobilizing biomolecules, drugs and synthetic polymers |
160
The following patents and patent applications (including expected 20 year expiration dates) relate to our immunofluorescence assay related products and technology.
| Patent Application Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US Patent App. No. 15/970,100, and foreign applications in certain jurisdictions claiming priority to PCT/US2018/030799 | Owned | May 3, 2038 | Directed to kits and methods related to immunofluorescence assays | |||
| US Patent App. No. 16/195,208, and foreign applications in certain jurisdictions claiming priority to PCT/US2018/061807 | Owned | Nov. 19, 2038 | Directed to systems and methods for immunoassay detection |
The following patents and patent applications (including expected 20 year expiration dates) relate to our immunofluorescence assay related products and technology.
| Patent Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 6,770,754 | Owned | Nov. 29, 2021 | Directed to compositions and methods related to oligonucleotide synthesis | |||
| US 7,491,817 | Owned | Nov. 29, 2021 | Directed to compositions and methods related to oligonucleotide synthesis | |||
| EP Patent No. 1404695 (validated in BE, CH, DE, FR, GB) | Owned | Nov. 29, 2021 | Directed to compositions and methods related to oligonucleotide synthesis | |||
| EP Patent No. 2248820 (validated in BE, CH, DE, FR, GB) | Owned | Nov. 29, 2021 | Directed to compositions and methods related to oligonucleotide synthesis |
| Patent Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 8,394,948 |
Co-owned with Nelson Biotechnologies | Sept. 28, 2030 | Directed to compositions and methods related to oligonucleotide synthesis |
| Patent Numbers |
Form of Ownership |
Expected Expiration Date |
Description | |||
| US 7,144,995 |
Co-owned with Berry & Associates | June 1, 2024 | Directed to compositions and methods related to fluorescent nitrogenous bases | |||
| EP Patent No. 1483280 (validated in CH, DE, FR, GB) | Co-owned with Berry & Associates | Mar. 6, 2023 | Directed to compositions and methods related to fluorescent nitrogenous bases |
PCT patent applications are not eligible to become an issued patent until, among other things, we file one or more national stage patent applications within, depending on the country, 30 to 32 months of the PCT application’s priority date in the countries in which we seek patent protection. Moreover, we may own
161
provisional patent applications in the future, and provisional patent applications are not eligible to become issued patents until, among other things, we file a non-provisional patent application within 12 months of filing of one or more of related provisional patent applications. If we do not timely file any national stage patent applications or non-provisional patent applications, we may lose our priority date with respect to our PCT patent applications or provisional patent applications and any patent protection on the inventions disclosed in such patent applications. While we intend to timely file national stage patent applications relating to our PCT patent applications and non-provisional patent applications relating to our provisional patent applications, we cannot predict whether any such patent applications will result in the issuance of patents that provide us with any competitive advantage.
Individual issued patents extend for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, utility patents issued for applications filed in the United States are granted a term of 20 years from the earliest effective filing date of a non-provisional patent application. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Trademarks. Our trademark portfolio is designed to protect the brands of our current and future products and includes U.S. trademark registrations for our company name, Maravai, and various product names, such as CleanCap®.
Trade Secrets. We also rely on trade secrets, including know-how, unpatented technology and other proprietary information, to strengthen our competitive position. We have determined that certain technologies, such as the production of antibodies for biologics safety testing, are better kept as trade secrets, rather than pursuing patent protection. To prevent disclosure of trade secrets to others, it is our policy to enter into nondisclosure, invention assignment and confidentiality agreements with parties who have access to trade secrets, such as our employees, collaborators, outside scientific collaborators, consultants, advisors and other third parties. These agreements also provide that all inventions resulting from work performed for us or relating to our business and conceived or completed during the period of employment or assignment, as applicable, are our exclusive property. In addition, we take other appropriate precautions, such as physical and technological security measures, to guard against misappropriation of our proprietary information by third parties.
We intend to pursue additional intellectual property protection to the extent we believe it would advance our business objectives. Notwithstanding these efforts, there can be no assurance that we will adequately protect our intellectual property or provide any competitive advantage. We cannot provide any assurance that any patents will be issued from our pending or any future patent applications or that any issued patents will adequately protect our products or technology. Our intellectual property rights may be invalidated, held unenforceable, circumvented, narrowed or challenged. In addition, the laws of various foreign countries where our products are distributed may not protect our intellectual property rights to the same extent as laws in the United States. Furthermore, it may be difficult to protect our trade secrets. While we have confidence in the measures we take to protect and preserve our trade secrets, they may be inadequate and can be breached, and we may not have adequate remedies for violations of such measures. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. Moreover, our invention assignment agreements with employees, collaborators, outside scientific collaborators, consultants, advisors and other third parties may not be self-executing or otherwise provide meaningful protection for our intellectual property rights. If we do not adequately protect our intellectual property, third parties, including our competitors, may be able to use our technologies to produce and market products that compete with us and erode our competitive advantage. For more information regarding risks related to intellectual property, please see “Risk Factors—Risks Related to our Intellectual Property.”
162
Facilities
Our corporate headquarters are in San Diego, California, where we occupy approximately 119,000 square feet of leased space. We completed construction of the facility in 2020. In addition to housing our headquarters, the facility serves as the principal hub of operations for our nucleic acid production business and was purpose built to expand the capacity of this business segment while adding specialized capabilities in the form of clean rooms, air handling, waste and solvent handling, and GMP capabilities.
Our other facilities are in Burlingame, California, Southport, North Carolina and Sterling, Virginia. Across all of our facilities we have approximately 140,000 square feet of lab and production space. All facilities are leased. Our facility in Burlingame is subject to a lease that terminates in June 2022, at which time we intend to relocate the operations. A summary of our facilities is listed below.
We own a 3,000 square foot facility in Peterborough, United Kingdom, which previously housed our local sales office. The building is being marketed for sale.
| Location |
Approx. Square Footage |
Products Produced |
Lease Term | |||
| San Diego, CA |
119,000 | TriLink BioTechnologies branded reagents | May 2030 | |||
| Burlingame, CA |
65,000 | Vector Laboratories branded reagents | June 2022 | |||
| Southport, NC |
20,000 | Cygnus Technologies branded reagents | July 2027 | |||
| Sterling, VA |
21,000 | Glen Research branded reagents | April 2025 |
Human Capital Resources
As of September 30, 2020, we had over 390 full-time employees. Among our employees, 43% identify as female and 57% identify as male. None of our employees is represented by a labor union, and none of our employees has entered into a collective bargaining agreement with us. We offer a highly competitive compensation and benefits program to attract and retain top talent.
Our talented employees drive our mission and share core values that both stem from and define our culture, which plays an invaluable role in our execution at all levels in our organization. Our culture is based on these shared core values which we believe contribute to our success and the continued growth of the organization. Our core values are used in candidate screening and in employee evaluations to help reinforce their importance in our organization:
| • | Adaptability. We stay agile, ready to shift or change our approach when challenges arise and open to new ideas and responsibilities. |
| • | Open Communication. We focus on open source sharing with our focus on constant improvement as individuals and as an organization. |
| • | Quality Mindset. We strive to eliminate errors with accurate work as a priority and seek opportunities to improve products/services. |
| • | Work Together. We are accountable to our team and work to meet established deliverables with respect and appreciation of others’ views. |
| • | Workplace Awareness. We promote a safe and healthy work environment. |
| • | Reward. We celebrate and recognize accomplishments, both individual and collectively. |
Legal Proceedings
From time to time, we may be involved in various legal proceedings and subject to claims that arise in the ordinary course of business. Although the results of litigation and claims are inherently unpredictable and uncertain, we are not currently a party to any legal proceedings the outcome of which, if determined adversely to
163
us, are believed to, either individually or taken together, have a material adverse effect on our business, operating results, cash flows or financial condition. Regardless of the outcome, litigation has the potential to have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors. See “Risk Factors—Risks Related to Our Intellectual Property—Intellectual property litigation and other proceedings could cause us to spend substantial resources and distract our personnel from their normal responsibilities” and “Risk Factors—Risks Related to Our Intellectual Property—If we are sued for infringing, misappropriating, or otherwise violating intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing our current or future products.”
164
Overview
Maravai LifeSciences Holdings, Inc. is a Delaware corporation formed to serve as a holding company that will hold an interest in Topco LLC. Maravai LifeSciences Holdings, Inc. has not engaged in any business or other activities other than in connection with its formation and this offering. Upon consummation of this offering and the application of the net proceeds therefrom, we will be a holding company, our sole asset will be an equity interest in Topco LLC and we will operate and control all of the business and affairs and consolidate the financial results of Topco LLC. Prior to the closing of this offering, the operating agreement of Topco LLC will be amended and restated to, among other things, modify its capital structure by replacing the membership interests currently held by Topco LLC’s existing owner, MLSH 1, with a new class of LLC Units. We and MLSH 1 will also enter into an Exchange Agreement under which MLSH 1 (and certain permitted transferees thereof) may (subject to the terms of the Exchange Agreement) exchange its LLC Units for shares of our Class A common stock on a one-for-one basis, or, at our election, for cash, from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). MLSH 1 will also be required to deliver to us an equivalent number of shares of Class B common stock to effectuate an exchange. Any shares of Class B common stock so delivered will be cancelled. As MLSH 1 exchanges its LLC Units, our interest in Topco LLC will be correspondingly increased.
Upon completion of this offering, GTCR will control the voting power in Maravai LifeSciences Holdings, Inc. as follows: (i) approximately 68% (or approximately 66% if the underwriters exercise their option to purchase additional shares in full) through its control of MLSH 1 and (ii) approximately 12% through its control of MLSH 2.
Incorporation of Maravai LifeSciences Holdings, Inc.
Maravai LifeSciences Holdings, Inc. was incorporated in Delaware on August 25, 2020, and has not engaged in any business or other activities except in connection with its formation and the offering. Our certificate of incorporation will be amended and restated at or prior to the consummation of this offering. Our amended and restated certificate of incorporation will authorize two classes of common stock, Class A common stock and Class B common stock, each having the terms described in “Description of Capital Stock.” In addition, our amended and restated certificate of incorporation will authorize shares of undesignated preferred stock, the rights, preferences and privileges of which may be designated from time to time by our Board.
Shares of our Class B common stock, which provide no economic rights, will be distributed to MLSH 1 in connection with this offering. Each share of our Class B common stock entitles its holder to one vote on all matters to be voted on by shareholders generally. See “Description of Capital Stock—Class B Common Stock.” Holders of our Class A common stock and Class B common stock vote together as a single class on all matters presented to our shareholders for their vote or approval, except as otherwise required by applicable law.
Organizational Transactions
The following transactions, referred to collectively herein as the “Organizational Transactions,” will each be completed prior to or in connection with the completion of this offering.
Immediately prior to the effectiveness of this Registration Statement, we will take the following actions:
| • | We will amend and restate the LLC Operating Agreement of Topco LLC to, among other things (i) modify its capital structure by replacing the membership interests currently held by Topco LLC’s existing owners (beneficially owned through MLSH 1) with a new class of LLC Units held initially by MLSH 1 and Maravai LifeSciences Holdings, Inc. and (ii) appoint Maravai LifeSciences Holdings, Inc. as the sole managing member of Topco LLC. |
165
| • | The Blocker Entities through which GTCR and Newstone hold a portion of their ownership interests in MLSH 1 will engage in a series of transactions that will result in each of these entities merging with and into Maravai LifeSciences Holdings, Inc., with Maravai LifeSciences Holdings, Inc. remaining as the surviving corporation. As a result of such transactions, MLSH 2 will exchange all of the equity interests in the Blocker Entities for shares of Maravai LifeSciences Holdings, Inc. Class A common stock. |
| • | We will amend and restate the certificate of incorporation of Maravai LifeSciences Holdings, Inc. to, among other things, provide for Class A common stock and Class B common stock. See “Description of Capital Stock.” |
| • | We will issue shares of Class B common stock to MLSH 1, on a one-to-one basis with the number of LLC Units it owns, for nominal consideration. |
| • | We expect to award (i) options to purchase an aggregate of 1,584,400 shares of Class A common stock with an exercise price set at the initial public offering price and (ii) 75,294 RSUs that may be settled for an equal number of shares of Class A common stock, each issued pursuant to the 2020 Plan. |
| • | We will enter into the Exchange Agreement with MLSH 1 pursuant to which MLSH 1 will be entitled to exchange LLC Units, together with an equal number of shares of Class B common stock, for shares of Class A common stock on a one-for-one basis or, at our election, for cash, from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). See “—Exchange Agreement.” |
| • | We will enter into the Tax Receivable Agreement with MLSH 1 and MLSH 2 that will provide for the payment by Maravai LifeSciences Holdings, Inc. to MLSH 1 and MLSH 2, collectively, of 85% of the amount of cash savings, if any, in U.S. federal, state and local income taxes (computed using simplifying assumptions to address the impact of state and local taxes) we actually realize (or under certain circumstances are deemed to realize in the case of an early termination payment by us, a change in control or a material breach by us of our obligations under the Tax Receivable Agreement, as discussed below) as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement. See “—Tax Receivable Agreement.” |
In connection with the completion of this offering, we will issue 50,000,000 shares of our Class A common stock to the investors in this offering (or 57,500,000 shares if the underwriters exercise their option to purchase additional shares in full) in exchange for net proceeds of approximately $1,184.5 million (or approximately $1,365.2 million if the underwriters exercise their option to purchase additional shares in full), after deducting underwriting discounts and commissions but before estimated offering expense payable by us.
Immediately following the completion of this offering, we will take the following actions:
| • | We will (i) use approximately $94.5 million of the net proceeds of this offering to acquire 3,921,569 newly-issued LLC Units in Topco LLC and approximately $942.7 million of the net proceeds of this offering to acquire 39,121,430 outstanding LLC Units in Topco LLC from MLSH 1 (or approximately $1,096.2 million to acquire 45,489,067 outstanding LLC units if the underwriters exercise their option to purchase additional shares in full), in each case at a purchase price per LLC Unit equal to the initial offering price per share of Class A common stock in this offering, less underwriting discounts and commissions and (ii) pay $167.6 million to MLSH 2 as consideration for the Blocker Mergers and, if the underwriters exercise their option to purchase additional shares in full, use $27.3 million to acquire outstanding shares of Class A common stock from MLSH 2. |
166
| • | Topco LLC will apply the proceeds it receives from us (including any additional proceeds it may receive from us if the underwriters exercise their option to purchase additional shares) to pay expenses incurred in connection with this offering and the Organizational Transactions and for general corporate purposes. Topco LLC will bear or reimburse us for all of the expenses of this offering, including the underwriters’ discounts and commissions. See “Use of Proceeds.” |
As a result of the Organizational Transactions:
| • | the investors in this offering will collectively own 50,000,000 shares of our Class A common stock and we will hold 81,379,818 LLC Units; |
| • | MLSH 1 will own 176,458,692 LLC Units and 176,458,692 shares of Class B common stock; |
| • | our Class A common stock will collectively represent approximately 32% of the voting power in us; and |
| • | our Class B common stock will collectively represent approximately 68% of the voting power in us. |
The diagram below depicts our historical organizational structure prior to the completion of the Organizational Transactions. This diagram is provided for illustrative purposes only and does not purport to represent all legal entities owned or controlled by us, or owning a beneficial interest in us.

167
The diagram below depicts our expected organizational structure immediately following completion of the Organizational Transactions. This diagram is provided for illustrative purposes only and does not purport to represent all legal entities owned or controlled by us, or owning a beneficial interest in us.

| (1) | Upon completion of this offering, GTCR will control the voting power in Maravai LifeSciences Holdings, Inc. as follows: (i) approximately 68% (or approximately 66% if the underwriters exercise their option to purchase additional shares in full) through its control of MLSH 1 and (ii) approximately 12% through its control of MLSH 2. See “Principal Shareholders” for additional information about MLSH 1 and MLSH 2. |
| (2) | Shares of Class A common stock and Class B common stock will vote as a single class. Each outstanding share of Class A common stock and Class B Common stock will be entitled to one vote on all matters to be voted on by shareholders generally. The shares of Class B common stock have no economic rights. In accordance with the Exchange Agreement to be entered into in connection with the Organizational Transactions, MLSH 1 will be entitled to exchange LLC Units, together with an equal number of shares of Class B common stock, for shares of Class A common stock determined in accordance with the Exchange Agreement or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). |
| (3) | Upon completion of this offering, we expect to award (i) options to purchase an aggregate of 1,584,400 shares of Class A common stock with an exercise price set at the initial public offering price and (ii) 75,294 RSUs that may be settled for an equal number of shares of Class A common stock, each issued pursuant to the 2020 Plan. |
| (4) | Assumes no exercise of the underwriters’ option to purchase additional shares. If the underwriters exercise their option to purchase additional shares in full, (i) the holders of Class A common stock will have 34% of the voting power in Maravai LifeSciences Holdings, Inc., with 12% held by MLSH 2, (ii) MLSH 1, through ownership of the Class B common stock, will have 66% of the voting power of Maravai LifeSciences |
168
| Holdings, Inc., (iii) MLSH 1 will own 66% of the outstanding LLC Units in Topco LLC and (iv) Maravai LifeSciences Holdings, Inc. will own 34% of the outstanding LLC Units in Topco LLC. |
Following the consummation of the Organizational Transactions, Maravai LifeSciences Holdings, Inc. will be a holding company and its sole asset will be its direct equity interest in Topco LLC. Maravai LifeSciences Holdings, Inc. will operate and control all of the business and affairs of Topco LLC and its subsidiaries. Accordingly, although Maravai LifeSciences Holdings, Inc. will initially own a minority economic interest in Topco LLC following the consummation of this offering, Maravai LifeSciences Holdings, Inc. will have 100% of the voting power and will control management of Topco LLC, subject to certain exceptions. The financial results of Topco LLC and its consolidated subsidiaries will be consolidated in our financial statements.
Our post-offering organizational structure will allow MLSH 1 to retain its equity ownership in Topco LLC, an entity that is classified as a partnership for United States federal income tax purposes, in the form of LLC Units. Investors in this offering will, by contrast, hold their equity ownership in Maravai LifeSciences Holdings, Inc., a Delaware corporation that is a domestic corporation for United States federal income tax purposes, in the form of shares of Class A common stock. We believe that MLSH 1 generally will find it advantageous to hold its equity interests in an entity that is not taxable as a corporation for United States federal income tax purposes. The LLC Unitholders, like Maravai LifeSciences Holdings, Inc., will be allocated their proportionate share of any taxable income of Topco LLC.
MLSH 1 will also hold shares of our Class B common stock. Although these shares of Class B common stock have only voting and no economic rights, they will allow MLSH 1 to exercise voting power over Maravai LifeSciences Holdings, Inc., the sole managing member of Topco LLC, at a level that is greater than their overall equity ownership of our business. Class B common stock is entitled to one vote per share. When MLSH 1 exchanges LLC Units for shares of our Class A common stock or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale), pursuant to the Exchange Agreement described below, it will also be required to deliver an equivalent number of shares of Class B common stock. Any shares of Class B common stock so delivered will be cancelled.
Amended and Restated Operating Agreement of Topco LLC
In connection with the completion of this offering, we will amend and restate Topco LLC’s existing operating agreement, which we refer to as the “LLC Operating Agreement.” The operations of Topco LLC, and the rights and obligations of the LLC Unitholders, will be set forth in the LLC Operating Agreement. The LLC Operating Agreement will be filed as an exhibit to the registration statement of which this prospectus forms a part.
Sole Manager
In connection with this offering, we will become a member and the sole managing member of Topco LLC. As the sole managing member, we will be able to control all of the day-to-day business affairs and decision-making of Topco LLC without the approval of any other member, unless otherwise stated in the LLC Operating Agreement. As such, through our officers and directors, we will be responsible for all operational and administrative decisions of Topco LLC and the day-to-day management of Topco LLC’s business. Pursuant to the LLC Operating Agreement, we cannot be removed, under any circumstances, as the sole managing member of Topco LLC except by our election.
Compensation
We will not be entitled to compensation for our services as managing member. We will be entitled to reimbursement by Topco LLC for fees and expenses incurred on behalf of Topco LLC, including all expenses associated with this offering and maintaining our corporate existence.
169
Recapitalization
The LLC Operating Agreement recapitalizes the interests currently held by the existing owner of Topco LLC, MLSH 1, into a new single class of common membership units, which we refer to as the “LLC Units.” The LLC Operating Agreement will also reflect a split of LLC Units such that one LLC Unit can be acquired with the net proceeds received in the initial offering from the sale of one share of our Class A common stock. Each LLC Unit will entitle the holder to a pro rata share of the net profits and net losses and distributions of Topco LLC. Holders of LLC Units will have no voting rights, except as expressly provided in the LLC Operating Agreement.
Distributions
The LLC Operating Agreement will require “tax distributions,” as that term is defined in the LLC Operating Agreement, to be made by Topco LLC to its “members,” as that term is defined in the LLC Operating Agreement. Tax distributions generally will be made quarterly to each member of Topco LLC, including us, on a pro rata basis among the LLC Unitholders based on Topco LLC’s net taxable income and without regard to any applicable basis adjustment under Section 743(b) of the Code and at a tax rate that will be determined by us. The tax rate used to determine tax distributions will apply regardless of the actual final tax liability of any such member. Tax distributions will also be made only to the extent all distributions from Topco LLC for the relevant period were otherwise insufficient to enable each member to cover its tax liabilities as calculated in the manner described above. We expect Topco LLC may make distributions out of distributable cash periodically to the extent permitted by agreements governing indebtedness of Topco LLC and necessary to enable Topco LLC to cover its operating expenses and other obligations, including our tax liability and obligations under the Tax Receivable Agreement, as well as to make dividend payments, if any, to the holders of our Class A common stock.
Exchange Rights
The LLC Operating Agreement provides that MLSH 1 (and certain permitted transferees thereof) may, pursuant to the terms of the Exchange Agreement described below, exchange its LLC Units for shares of our Class A common stock on a one-for-one basis, or, at our election, for cash, from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). MLSH 1 will also be required to deliver to us an equivalent number of shares of Class B common stock to effectuate an exchange. As a holder surrenders or exchanges its LLC Units, our interest in Topco LLC will be correspondingly increased. See “—Exchange Agreement.”
Issuance of LLC Units Upon Exercise of Options or Issuance of Other Equity Compensation
Upon the exercise of options issued by us, or the issuance of other types of equity compensation by us (such as the issuance of restricted or non-restricted stock, payment of bonuses in stock or settlement of stock appreciation rights in stock), we will be required to acquire from Topco LLC a number of LLC Units equal to the number of shares of Class A common stock being issued in connection with the exercise of such options or issuance of other types of equity compensation. When we issue shares of Class A common stock in settlement of stock options granted to persons that are not officers or employees of Topco LLC or its subsidiaries, we will make, or be deemed to make, a capital contribution to Topco LLC equal to the aggregate value of such shares of Class A common stock, and Topco LLC will issue to us a number of LLC Units equal to the number of shares of Class A common stock we issued. When we issue shares of Class A common stock in settlement of stock options granted to persons that are officers or employees of Topco LLC or its subsidiaries, we will be deemed to have sold directly to the person exercising such award a portion of the value of each share of Class A common stock equal to the exercise price per share, and we will be deemed to have sold directly to Topco LLC (or the applicable subsidiary of Topco LLC) the difference between the exercise price and market price per share for each such share of Class A common stock. In cases where we grant other types of equity compensation to employees of Topco LLC or its subsidiaries, on each applicable vesting date we will be deemed to have sold to
170
Topco LLC (or such subsidiary) the number of vested shares of Class A common stock at a price equal to the market price per share, Topco LLC (or such subsidiary) will deliver the shares to the applicable person, and we will be deemed to have made a capital contribution in Topco LLC equal to the purchase price for such shares in exchange for an equal number of LLC Units.
Maintenance of One-to-One Ratio of Shares of Class A Common Stock and LLC Units Owned by Maravai LifeSciences Holdings, Inc.
Our amended and restated certificate of incorporation and the LLC Operating Agreement will require that (1) we at all times maintain a ratio of one LLC Unit owned by us for each share of Class A common stock issued by us (subject to certain exceptions for treasury shares and shares underlying certain convertible or exchangeable securities), and (2) Topco LLC at all times maintains a one-to-one ratio between the number of shares of Class A common stock issued by us and the number of LLC Units owned by us.
Transfer Restrictions
The LLC Operating Agreement generally does not permit transfers of LLC Units by members, subject to limited exceptions. Any transferee of LLC Units must assume, by operation of law or written agreement, all of the obligations of a transferring member with respect to the transferred units, even if the transferee is not admitted as a member of Topco LLC.
Dissolution
The LLC Operating Agreement will provide that the unanimous consent of all members holding voting units will be required to voluntarily dissolve Topco LLC. In addition to a voluntary dissolution, Topco LLC will be dissolved upon a change of control transaction under certain circumstances, as well as upon the entry of a decree of judicial dissolution or other circumstances in accordance with Delaware law. Upon a dissolution event, the proceeds of a liquidation will be distributed in the following order: (1) first, to pay the expenses of winding up Topco LLC; (2) second, to pay debts and liabilities owed to creditors of Topco LLC, other than members; (3) third, to pay debts and liabilities owed to members; and (4) fourth, to the members pro rata in accordance with their respective percentage ownership interests in Topco LLC (as determined based on the number of LLC Units held by a member relative to the aggregate number of all outstanding LLC Units).
Confidentiality
Each member will agree to maintain the confidentiality of Topco LLC’s confidential information. This obligation excludes information independently obtained or developed by the members, information that is in the public domain or otherwise disclosed to a member, in either such case not in violation of a confidentiality obligation or disclosures required by law or judicial process or approved by our chief executive officer.
Indemnification and Exculpation
The LLC Operating Agreement provides for indemnification of the manager, members and officers of Topco LLC and their respective subsidiaries or affiliates. To the extent permitted by applicable law, Topco LLC will indemnify us, as its managing member, its authorized officers, its other employees and agents from and against any losses, liabilities, damages, costs, expenses, fees or penalties incurred by any acts or omissions of these persons, provided that the acts or omissions of these indemnified persons are not the result of fraud, intentional misconduct or a violation of the implied contractual duty of good faith and fair dealing, or any lesser standard of conduct permitted under applicable law.
We, as the managing member, and the authorized officers and other employees and agents of Topco LLC will not be liable to Topco LLC, its members or their affiliates for damages incurred by any acts or omissions of
171
these persons, provided that the acts or omissions of these exculpated persons are not the result of fraud, or intentional misconduct.
Amendments
The LLC Operating Agreement may be amended with the consent of the holders of a majority in voting power of the outstanding LLC Units. Notwithstanding the foregoing, no amendment to any of the provisions that expressly require the approval or action of certain members may be made without the consent of such members and no amendment to the provisions governing the authority and actions of the managing member or the dissolution of Topco LLC may be amended without the consent of the managing member.
Tax Receivable Agreement
The purchase of LLC Units by us in connection with this offering is expected to result in the acquisition by us of a proportionate share of the existing tax basis of the assets of Topco LLC and its flow-through subsidiaries. Topco LLC (and each of its subsidiaries classified as a partnership for U.S. federal income tax purposes) intends to have in place for its taxable year in which this offering and the associated purchase of LLC Units occurs an election under Section 754 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”). Accordingly, such purchase of LLC Units by us is expected to result in an adjustment in the tax basis of the assets of Topco LLC and its flow-through subsidiaries reflected in the proportionate share of such assets treated as acquired by us.
In addition, MLSH 1 may from time to time (subject to the terms of the Exchange Agreement) exercise a right to exchange LLC Units for shares of our Class A common stock on a one-for-one basis, or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). We intend to treat such acquisitions of LLC Units as direct purchases of LLC Units from MLSH 1 for U.S. federal income and other applicable tax purposes, regardless of whether such LLC Units are surrendered by MLSH 1 to Topco LLC for redemption or sold to us upon the exercise of our election to acquire such LLC Units directly. Topco LLC (and each of its subsidiaries classified as a partnership for U.S. federal income tax purposes) intends to have in place an election under Section 754 of the Code effective for each taxable year in which an exchange of LLC Units for Class A common stock or cash occurs. As a result, an exchange of LLC Units is expected to result in (1) an increase in our proportionate share of the existing tax basis of the assets of Topco LLC and its flow-through subsidiaries and (2) an adjustment in the tax basis of the assets of Topco LLC and its flow-through subsidiaries reflected in that proportionate share (“Basis Adjustments”).
Any increases in our share of tax basis as a result of the purchase of LLC Units or LLC Unit exchanges will generally have the effect of reducing the amounts that we would otherwise be obligated to pay thereafter to various tax authorities. Such basis increases may also decrease gains (or increase losses) on future dispositions of certain assets to the extent tax basis is allocated to those assets.
As a result of the mergers of the Blocker Mergers, we will succeed to the federal net operating losses (“NOL”) and certain other existing tax attributes of the Blocker Entities. Subject to certain limitations, such NOLs and other attributes may be available to offset our taxable income in future years (and in certain circumstances, taxable income from prior years) in the manner described below.
An NOL occurs when a taxpayer’s tax deductions exceed its taxable income within a given tax year. An NOL can be carried forward over future tax years and used to offset taxable income incurred in such future tax year. The 2017 tax reform legislation known as the Tax Cuts and Jobs Act of 2017 lifted the previous 20-year limitation on NOL carryforwards (allowing NOLs to be carried forward indefinitely), but limited NOLs to 80% of taxable income in any one tax period. Notably, among other changes, the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”) has temporarily removed this 80% limit for taxable years beginning
172
before 2021 to allow an NOL carryforward to fully offset a taxpayer’s income, and additionally, to allow NOLs incurred in 2018, 2019, and 2020 to be carried back to offset taxable income up to five years prior to the taxable year in which the NOL was generated.
We intend to enter into a Tax Receivable Agreement with MLSH 1 and MLSH 2. The Tax Receivable Agreement provides for the payment by us to MLSH 1 and MLSH 2, collectively, of 85% of the amount of tax benefits, if any, that we actually realize, or in some circumstances are deemed to realize, as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement (collectively, the “Tax Attributes”). The payment obligations under the Tax Receivable Agreement are not conditioned upon any LLC Unitholder maintaining a continued ownership interest in us or Topco LLC and the rights of MLSH 1 under the Tax Receivable Agreement are assignable. We expect to benefit from the remaining 15% of the tax benefits, if any, that we may actually realize.
For purposes of the Tax Receivable Agreement, the tax benefit deemed realized by us will generally be computed by comparing our actual cash income tax liability to the amount of such taxes that we would have been required to pay had there been no Tax Attributes; provided that, for purposes of determining the tax benefit with respect to state and local income taxes, we will use simplifying assumptions. The Tax Receivable Agreement will generally apply to each of our taxable years, beginning with the taxable year that the Tax Receivable Agreement is entered into. There is no maximum term for the Tax Receivable Agreement and the Tax Receivable Agreement will continue until all such tax benefits have been utilized or expired unless we exercise our right to terminate the Tax Receivable Agreement for an agreed-upon amount equal to the estimated present value of the remaining payments to be made under the agreement (calculated with certain assumptions, including as to utilization of the Tax Attributes).
The actual Tax Attributes, as well as any amounts paid to MLSH 1 and MLSH 2 under the Tax Receivable Agreement, will vary depending on a number of factors, including:
| • | the timing of any future exchanges—for instance, the increase in any tax deductions will vary depending on the fair value, which may fluctuate over time, of the depreciable or amortizable assets of Topco LLC and its flow-through subsidiaries at the time of each exchange; |
| • | the price of shares of our Class A common stock at the time of any future exchanges—the Basis Adjustments are directly related to the price of shares of our Class A common stock at the time of future exchanges; |
| • | the extent to which such exchanges are taxable—if an exchange is not taxable for any reason, increased tax deductions as a result of the Section 754 election mentioned above will not be available to generate payments under the Tax Receivable Agreement; |
| • | the amount and timing of our income—the Tax Receivable Agreement generally will require us to pay 85% of the tax benefits as and when those benefits are treated as realized by us under the terms of the Tax Receivable Agreement. If we do not have taxable income in a particular taxable year, we generally will not be required (absent a change of control or other circumstances requiring an early termination payment) to make payments under the Tax Receivable Agreement for that taxable year because no tax benefits will have been actually realized. Nevertheless, any tax benefits that do not result in realized tax benefits in a given taxable year will likely generate tax attributes that may be utilized to generate tax benefits in future (and possibly previous) taxable years. The utilization of any such tax attributes will result in payments under the Tax Receivable Agreement; and |
| • | applicable tax rates—the tax rates in effect at the time a tax benefit is recognized. |
173
The payment obligations under the Tax Receivable Agreement are obligations of Maravai LifeSciences Holdings, Inc. and not of Topco LLC. Although the actual timing and amount of any payments that may be made under the Tax Receivable Agreement will vary, we expect that the aggregate payments that we will be required to make to MLSH 1 and MLSH 2 will be substantial. Any payments made by us under the Tax Receivable Agreement will generally reduce the amount of overall cash flow that might have otherwise been available to us or to Topco LLC and, to the extent that we are unable to make payments under the Tax Receivable Agreement for any reason, the unpaid amounts will be deferred and will accrue interest until paid by us. We anticipate funding ordinary course payments under the Tax Receivable Agreement from cash flow from operations of Topco LLC and its subsidiaries, available cash and/or available borrowings under the New Credit Agreement.
We expect that the aggregate payments that we may make under the Tax Receivable Agreement will be substantial. Assuming no material changes in the relevant tax law, and that we earn sufficient taxable income to realize all tax benefits that are subject to the Tax Receivable Agreement, we expect that future payments under the Tax Receivable Agreement relating to the purchase by Maravai LifeSciences Holdings, Inc. of LLC Units from MLSH 1 in connection with this offering to be approximately $279.8 million (or approximately $317.8 million if the underwriters exercise their option to purchase additional shares (the proceeds of which will be used by Maravai LifeSciences Holdings, Inc. to acquire additional LLC Units from MLSH 1) and to range over the next 15 years from approximately $5.3 to $21.2 million per year (or range from approximately $6.0 to $24.1 million per year if the underwriters exercise their option to purchase additional shares) and decline thereafter. As a result, we expect that aggregate payments under the Tax Receivable Agreement over this 15-year period will be approximately $279.8 million (or approximately $317.8 million if the underwriters exercise their option to purchase additional shares). These estimates are based on an initial public offering price of $25.50 per share of Class A common stock, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus. Future payments in respect of subsequent exchanges or financing would be in addition to these amounts and are expected to be substantial. The foregoing numbers are merely estimates—the actual payments could differ materially. It is possible that future transactions or events could increase or decrease the actual tax benefits realized and the corresponding Tax Receivable Agreement payments. There may be a material negative effect on our liquidity if, as a result of timing discrepancies or otherwise, the payments under the Tax Receivable Agreement exceed the actual benefits we realize in respect of the tax attributes subject to the Tax Receivable Agreement and/or distributions to Maravai LifeSciences Holdings, Inc. by Topco LLC are not sufficient to permit Maravai LifeSciences Holdings, Inc. to make payments under the Tax Receivable Agreement after it has paid taxes.
The Tax Receivable Agreement provides that if (1) certain mergers, asset sales, other forms of business combination, or other changes of control were to occur, (2) we materially breach any of our material obligations under the Tax Receivable Agreement or (3) we elect an early termination of the Tax Receivable Agreement, then the Tax Receivable Agreement will terminate and our obligations, or our successor’s obligations, under the Tax Receivable Agreement will accelerate and become due and payable, based on certain assumptions, including an assumption that we would have sufficient taxable income to fully utilize all potential future tax benefits that are subject to the Tax Receivable Agreement and, to the extent applicable, that any LLC Units that have not been exchanged are deemed exchanged for the fair market value of our Class A common stock at the time of termination.
As a result of a change of control, material breach, or our election to terminate the Tax Receivable Agreement early, (1) we could be required to make cash payments to MLSH 1 and MLSH 2 that are greater than the specified percentage of the actual benefits we ultimately realize in respect of the tax benefits that are subject to the Tax Receivable Agreement and (2) we will be required to make an immediate cash payment equal to the present value of the anticipated future tax benefits that are the subject of the Tax Receivable Agreement, which payment may be made significantly in advance of the actual realization, if any, of such future tax benefits. In these situations, our obligations under the Tax Receivable Agreement could have a material adverse effect on our liquidity and could have the effect of delaying, deferring or preventing certain mergers, asset sales, other forms
174
of business combination, or other changes of control. There can be no assurance that we will be able to finance our obligations under the Tax Receivable Agreement.
Payments under the Tax Receivable Agreement will be based on the tax reporting positions that we determine. Although we are not aware of any issue that would cause the IRS to challenge a tax basis increase or the availability of Blocker Entities’ NOLs, we will not be reimbursed for any cash payments previously made to MLSH 1 and MLSH 2 pursuant to the Tax Receivable Agreement if any tax benefits initially claimed by us are subsequently disallowed, in whole or in part, by the IRS or other applicable taxing authority. For example, if the IRS later asserts that we did not obtain a tax basis increase or disallows (in whole or in part) the availability of NOLs due to a potential ownership change under Section 382 of the Code, among other potential challenges, then we would not be reimbursed for any cash payments previously made to MLSH 1 and MLSH 2 pursuant to the Tax Receivable Agreement with respect to such tax benefits that we had initially claimed. Instead, any excess cash payments made by us pursuant to the Tax Receivable Agreement will be netted against any future cash payments that we might otherwise be required to make under the terms of the Tax Receivable Agreement. Nevertheless, any tax benefits initially claimed by us may not be disallowed for a number of years following the initial time of such payment or, even if challenged early, such excess cash payment may be greater than the amount of future cash payments that we might otherwise be required to make under the terms of the Tax Receivable Agreement. Accordingly, there may not be sufficient future cash payments against which to net. The applicable U.S. federal income tax rules are complex, and there can be no assurance that the IRS or a court will not disagree with our tax reporting positions. As a result, it is possible that we could make cash payments under the Tax Receivable Agreement that are substantially greater than our actual cash tax savings.
Under the Tax Receivable Agreement, we are required to provide MLSH 1 and MLSH 2 with a schedule setting forth the calculation of payments that are due under the Tax Receivable Agreement with respect to each taxable year in which a payment obligation arises within sixty (60) days after filing our U.S. federal income tax return for such taxable year. This calculation will be based upon the advice of our tax advisors. Payments under the Tax Receivable Agreement will generally be made within three (3) business days after this schedule becomes final pursuant to the procedures set forth in the Tax Receivable Agreement, although interest on such payments will begin to accrue at a rate of LIBOR plus 100 basis points from the due date (without extensions) of such tax return. Any late payments that may be made under the Tax Receivable Agreement will continue to accrue interest at LIBOR plus 100 basis points until such payments are made, generally including any late payments that we may subsequently make because we did not have enough available cash to satisfy our payment obligations at the time at which they originally arose.
Exchange Agreement
We will enter into the Exchange Agreement with MLSH 1. Under the Exchange Agreement, MLSH 1 (and certain permitted transferees thereof) may (subject to the terms of the Exchange Agreement) surrender their LLC Units to Topco LLC or, at our election, exchange its LLC Units for shares of our Class A common stock on a one-for-one basis, or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). MLSH 1 will also be required to deliver to us an equivalent number of shares of Class B common stock to effectuate an exchange. Any shares of Class B common stock so delivered will be cancelled. As a holder surrenders or exchanges its LLC Units, our interest in Topco LLC will be correspondingly increased.
Registration Rights Agreement
We intend to enter into the Registration Rights Agreement with MLSH 1 and MLSH 2 in connection with this offering. The Registration Rights Agreement will provide MLSH 1 and MLSH 2 certain registration rights whereby, following our initial public offering and the expiration of any related lock-up period, MLSH 1 and MLSH 2 can require us to register under the Securities Act shares of Class A common stock owned by them or issuable to MLSH 1 upon exchange of its LLC Units. The Registration Rights Agreement will also provide for piggyback registration rights for MLSH 1 and MLSH 2. See “Certain Relationships and Related Party Transactions—Registration Rights Agreement.”
175
Our Executive Officers and Directors
Below is a list of the names, ages as of September 30, 2020, positions and brief accounts of the business experience of the individuals who serve as (i) our executive officers, (ii) our directors and (iii) our director nominees. Upon the completion of this offering, Messrs. Cunningham, Daverman, Hance, Lucier, Marker and Mihas, Drs. Hopfield and Prahalad, Ms. Ashkenazi and Ms. Gray are anticipated to be elected to our Board.
| Name |
Age |
Position | ||
| Carl Hull |
62 |
Chief Executive Officer and Director | ||
| Eric Tardif |
51 |
President | ||
| Kevin Herde |
49 |
Chief Financial Officer | ||
| Brian Neel |
44 |
Chief Operating Officer, Nucleic Acid Production | ||
| Christine Dolan |
52 |
Chief Operating Officer, Biologics Safety Testing | ||
| Lisa Sellers |
48 |
Chief Operating Officer, Protein Detection | ||
| Kurt Oreshack |
40 |
General Counsel | ||
| Anat Ashkenazi |
48 |
Director Nominee | ||
| Sean Cunningham |
45 |
Director Nominee | ||
| Benjamin Daverman |
42 |
Director Nominee | ||
| Susannah Gray |
60 |
Director Nominee | ||
| Robert B. Hance |
61 |
Director Nominee | ||
| Jessica Hopfield |
55 |
Director Nominee | ||
| Gregory T. Lucier |
56 |
Director Nominee | ||
| Luke Marker |
35 |
Director Nominee | ||
| Constantine Mihas |
53 |
Director Nominee | ||
| Murali K. Prahalad |
49 |
Director Nominee | ||
Carl Hull has served as our Chief Executive Officer since he co-founded Maravai in March 2014. Mr. Hull brings over 35 years of sales, marketing and general management leadership in the diagnostics and life sciences industries. From 2009 to 2012, Mr. Hull was Chief Executive Officer of Gen-Probe Incorporated (“Gen-Probe”), a medical diagnostics company, and served as its Chief Operating Officer from 2007 to 2009. Under Mr. Hull’s leadership, Gen-Probe took full advantage of its core molecular diagnostics and automation strengths and launched several highly innovative products including the PANTHER® molecular diagnostic system and APTIMA® HPV screening assay. During Mr. Hull’s tenure, Gen-Probe extended its recognized leadership position in the most rapidly growing diagnostics market segment and the market capitalization of Gen-Probe doubled, creating nearly $2 billion in value for shareholders and culminating in a successful sale to Hologic in 2012. Prior to Gen-Probe, Mr. Hull had been in sales, marketing and management positions for Abbott Laboratories, Ventana Medical Systems, Inc. (acquired by Roche Holding AG), Applied Imaging Corp. (now part of Danaher Corporation) and Applied Biosystems Inc. (now part of Thermo Fisher Scientific Inc. (“Thermo Fisher”)), all biomedical technology companies. Mr. Hull serves as Chairman of the Board for The Binding Site and is a member of the Board of Ortho Clinical Diagnostics, both leading human diagnostics companies. Mr. Hull holds an MBA from the University of Chicago and a BA in Political Science and International Relations from the Johns Hopkins University.
Eric Tardif has served as our President since he co-founded Maravai in March 2014. Prior to co-founding Maravai, he led corporate development and corporate strategy at Gen-Probe Incorporated. Following the
176
acquisition of Gen-Probe by Hologic, Inc. (“Hologic”), a medical technology company, in 2012, Mr. Tardif was promoted to lead corporate strategy for Hologic. Mr. Tardif began his career as an investment banker executing mergers and acquisitions at investment banking firms Merrill Lynch, Piper Jaffray and Morgan Stanley, with a focus on medical device companies, particularly in the life sciences tools and diagnostics segments. Mr. Tardif has a Master of Science in Finance from Boston College, an MBA from University of British Columbia and a BA in Business Administration from Major Bishops University.
Kevin Herde has served as our Chief Financial Officer since May 2017. Prior to joining Maravai, he served as Executive Vice President and Chief Financial Officer at Sorrento Therapeutics, Inc., a biopharmaceutical company, from April 2016 to May 2017 and as Vice President of Global Blood Screening at Hologic from January 2015 to February 2016. Mr. Herde also served as Vice President, Finance and Corporate Controller at Gen-Probe prior to its acquisition by Hologic in 2012. Mr. Herde began his career at KPMG LLC. Mr. Herde holds a BBA in Business Administration from University of San Diego and is a certified public accountant in California (inactive).
Brian Neel has served as the Chief Operating Officer for our Nucleic Acid Production business segment since October 2017. Prior to joining Maravai, Mr. Neel was Vice President of Operations of Codex DNA, Inc. (formerly Synthetic Genomics DNA) (“Codex”), a biological equipment company, from May 2016 to October 2017. Prior to joining Codex, Mr. Neel was Vice President of Operations of GenMark Diagnostics, Inc. (“Genmark”), a molecular diagnostics company, from 2014 to 2016. Prior to joining GenMark, Mr. Neel was the Site Manufacturing Operations Leader at Thermo Fisher Scientific (formerly Life Technologies) from January 2013 to June 2014. Prior to joining Thermo Fisher, Mr. Neel was a Global Operations Associate Director and Manufacturing Operations Leader at Life Technologies, Inc. (“Life Technologies”), a global life sciences company that was ultimately purchased by Thermo Fisher in 2014, for over eleven years. Mr. Neel holds a BS in Microbiology from the University of Missouri.
Christine Dolan has served as the Chief Operating Officer of our Biologics Safety Testing business segment since October 2017. Prior to joining Maravai, Ms. Dolan was a Senior Vice President of Product Development at Catalent, Inc. (“Catalent”), a biotechnology company, where Ms. Dolan worked for over eight years. Prior to joining Catalent, Ms. Dolan was a Director of Nuclear Operations and Global Quality Control and a Director of QC, Validation and Supply Chain Management at GE Healthcare, a medical technology company, for over three years. Prior to joining GE Healthcare, Ms. Dolan was a Director of QC and Materials Management at Amersham, where she worked for over ten years. Ms. Dolan has more than 25 years of global leadership experience and successful profit-and-loss management of diverse businesses in the pharmaceutical industry. Ms. Dolan holds a BS in Biology from Lenoir-Rhyne College.
Lisa Sellers has served as the Chief Operating Officer of our Protein Detection business segment since August 2020. With over 20 years of experience, Lisa is an experienced general manager and commercial executive. Prior to joining Maravai, Lisa was Vice President of Marketing at 10X Genomics, Inc. (“10X”). Prior to 10X, Lisa led global reagent and instrumentation businesses within Applied Biosystems, Life Technologies and then Thermo Fisher Scientific. While at Thermo Fisher, Lisa also led B2B business development and sales channels to supply and out-license a portfolio of genetic analysis products and IP to the molecular diagnostic market. Lisa received her PhD in Chemistry from the University of Colorado at Boulder and her BS in Chemistry from Santa Clara University.
Kurt Oreshack has served as our General Counsel since November 2020. Prior to joining Maravai, Mr. Oreshack was a partner in the law firm of Breakwater Law Group, LLP, practicing in the field of corporate and securities law. Prior to joining Breakwater Law Group, Mr. Oreshack was the General Counsel of Human Longevity, Inc., a genomic research and in vitro diagnostics company, from June 2015 through September 2017. After leaving Human Longevity, Mr. Oreshack practiced law individually and as General Counsel in Residence at the law firm of Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP until joining Breakwater Law Group in January 2019. Mr. Oreshack received a JD from the University of Notre Dame Law School and a BA from Loyola University Chicago. He is a member of the State Bar of California.
177
Anat Ashkenazi will begin serving on our board upon the completion of this offering. Ms. Ashkenazi is a Senior Vice President of Finance at Eli Lilly and Company, where she has worked for over nineteen years. Ms. Ashkenazi joined Eli Lilly in 2001 and has had a diverse career spanning financial, strategy and operations roles. Prior to her current position, Ms. Ashkenazi held roles as the chief financial officer for a number of global divisions within Eli Lilly, including Oncology, Diabetes, Global Manufacturing & Quality and Research & Development. Ms. Ashkenazi holds an MBA from Tel Aviv University and a BA in Economics & Business Administration from the Hebrew University. We determined that Ms. Ashkenazi’s extensive executive experience in the pharmaceutical industry, as well as her financial expertise, qualifies her to serve as a director on the Board of Directors.
Sean Cunningham will begin serving on our board upon the completion of this offering and has served as a member of MLSH 1’s board since March 2016. Mr. Cunningham joined GTCR in 2001 where he is currently a Managing Director. He was previously a consultant with The Boston Consulting Group. Mr. Cunningham holds an MBA from the Wharton School at the University of Pennsylvania as well as a BA and BE in Engineering sciences from Dartmouth College. Mr. Cunningham served on the board of managers of MLSH 1 and will continue to serve until he is appointed as director in connection with the closing of the offering. We determined that Mr. Cunningham’s directorship experience with similar companies and extensive experience in the healthcare and pharmaceutical industries qualifies him to serve as a director on the Board of Directors.
Benjamin Daverman will begin serving on our board upon the completion of this offering and has served as a member MLSH 1’s board since March 2016. Mr. Daverman joined GTCR in 2008 where he is currently a Managing Director. Prior to joining GTCR, he worked as a Venture Capitalist at Alta Partners, a venture capital firm, as well as an Investment Banking Associate at JMP Securities and an analyst in the mergers and acquisitions group at J.P. Morgan (formerly Hambrecht & Quist), both investment banking firms. Mr. Daverman holds an MBA from the Wharton School at the University of Pennsylvania and a BA in History from Colgate University. He also holds an MS in Biotechnology from the School of Engineering and Applied Science at the University of Pennsylvania. Mr. Daverman served on the board of managers of MLSH 1 and will continue to serve until he is appointed as director in connection with the closing of the offering. We determined that Mr. Daverman’s extensive directorship experience with similar companies, and extensive experience in the healthcare, pharmaceutical and life sciences qualifies him to serve as a director on the Board of Directors.
Susannah Gray will begin serving on our board upon the completion of this offering. Ms. Gray served as the Chief Financial Officer of Royalty Pharma Management LLC (“Royalty Pharma”), a buyer of pharmaceutical royalties, from January 2005 to December 2018. She was promoted to Executive Vice President of Finance and Strategy in December 2018 and retired from Royalty Pharma in September 2019. Prior to Royalty Pharma, Ms. Gray served as a managing director and senior analyst covering the healthcare sector of CIBC World Markets’ high yield group from 2002 to 2004, and also previously served in similar roles at Merrill Lynch and Chase Securities (predecessor of J.P. Morgan Securities). Ms. Gray holds an MBA from Columbia University and a BA in Social Studies from Wesleyan University. We determined that Ms. Gray’s extensive executive experience in the pharmaceutical industry, as well as her financial expertise, qualifies her to serve as a director on the Board of Directors.
Robert B. Hance will begin serving on our board upon the completion of this offering and has served as a member of MLSH 1’s board since 2017. Mr. Hance is a medical device industry veteran with more than 25 years’ experience and has served as the Chief Executive Officer of Regatta Medical, Inc. (“Regatta Medical”), a medical device company, since 2017. Prior to Regatta Medical from 2013 to 2016, Mr. Hance was Chief Executive Officer of Creganna Medical Devices, Inc. (“Creganna Medical”), a leading supplier to the minimally invasive medical device industry. Creganna Medical was sold to TE Connectivity Ltd. in 2016. From 2012 to 2013, Mr. Hance was an Entrepreneur-in-Residence within the FDA at the Center for Devices and Radiological Health. Prior to his FDA experience, Mr. Hance was President of Abbott Vascular, the cardiovascular device division of Abbott Laboratories, a biomedical company. Mr. Hance holds an MBA from Harvard Business School and a BS in Chemical Engineering from the Massachusetts Institute of Technology. Mr. Hance served on the board of managers of MLSH 1 and will continue to serve until he is appointed as director in connection with
178
the closing of the offering. We determined Mr. Hance’s extensive expertise in the medical device and life sciences industry qualifies him to serve as a director on the Board of Directors.
Jessica Hopfield, PhD will begin serving on our board upon the completion of this offering. Dr. Hopfield is a scientist and business leader with more than two decades of experience in the medical and healthcare fields. She serves as an independent director on the Board of Directors of Insulet Corporation, Editas Medicine and Radius Health, Inc. In addition, she is a strategic advisor and investor in start-up healthcare firms. Dr. Hopfield is a former Partner of McKinsey & Company in its global pharmaceuticals and medical devices practice where she led work in strategy, research and development management, and marketing across pharmaceutical, biotechnology and medical device industries. She also held management positions at Merck Sharp & Dohme Corp., a pharmaceutical company, in clinical development, outcomes research, and marketing. Dr. Hopfield holds a PhD in Biological Sciences from The Rockefeller University, an MBA from Harvard Business School and a BS in Biology from Yale College. We determined that Dr. Hopfield is qualified to serve as a member of our Board because of her extensive experience in the life sciences industry, educational background and service as an independent director to other public companies.
Gregory T. Lucier will begin serving on our board upon the completion of this offering and has served as a member of MLSH 1’s board since January 2020. Mr. Lucier has served as the Chief Executive Officer of Corza Health, Inc. (“Corza Health”), a life sciences company, since 2018 and is a 25-year veteran of the healthcare industry. Prior to Corza Health, Mr. Lucier was Chairman and Chief Executive Officer of NuVasive, Inc. (“NuVasive”) from 2015 to 2018. NuVasive is an innovative medical device company specializing in minimally invasive spine surgery. Prior to NuVasive, from 2003 to 2014, Mr. Lucier served as Chairman and CEO of Life Technologies. Mr. Lucier’s early career included roles as a corporate officer of General Electric Company and as an executive at GE Medical Systems Information Technologies, Inc., a healthcare company. Mr. Lucier continues to serve as director and Chairman of NuVasive, as well as director of Catalent and Dentsply Sirona Inc., a global provider of professional dental products and technologies. He has an MBA from Harvard Business School and a BA in Industrial Engineering from Pennsylvania State University. Mr. Lucier served on the board of managers of MLSH 1 and will continue to serve until he is appointed as director in connection with the closing of the offering. We determined Mr. Lucier’s extensive experience in the healthcare and medical device industry, in addition to his experience on multiple public and private boards of directors, qualifies him to serve as a director on the Board of Directors.
Luke Marker will begin serving on our board upon the completion of this offering and has served as a member of MLSH 1’s board since 2016. Mr. Marker joined GTCR in 2009 and became a Principal in 2020. Prior to joining GTCR, he worked in the investment banking division at Lehman Brothers and Barclays Capital. Mr. Marker holds an MBA with distinction from Harvard Business School and a BA in Mathematics and Economics from Kalamazoo College. Mr. Marker served on the board of managers of MLSH 1 and will continue to serve until he is appointed as director in connection with the closing of the offering. We determined that Mr. Marker’s directorship experience with similar companies and extensive experience in the healthcare, pharmaceutical and life sciences industries qualifies him to serve as a director on the Board of Directors.
Constantine Mihas will begin serving on our board upon the completion of this offering and has served as a member MLSH 1’s board since March 2016. Mr. Mihas joined GTCR in 2001 where he is currently a Managing Director and head of the Healthcare group. Prior to joining GTCR, Mr. Mihas was Chief Executive Officer and co-founder of Delray Farms, LLC (“Delray Farms”), a specialty food retailer. Prior to Delray Farms, Mr. Mihas was with McKinsey & Company, Inc., a consulting firm. Mr. Mihas holds an MBA with distinction from the Harvard Business School and a BS in Finance and Economics from the University of Illinois, Chicago. Mr. Mihas served on the board of managers of MLSH 1 and will continue to serve until he is appointed as director in connection with the closing of the offering. We determined that Mr. Mihas’ directorship experience with similar companies, deep business background, and extensive experience in the healthcare, pharmaceutical and life sciences industries qualifies him to serve as a director on the Board of Directors.
Murali K. Prahalad will begin serving on our board upon the completion of this offering and has served as a member MLSH 1’s board since August 2016. Dr. Prahalad is currently the President and Chief Executive Officer of Iridia, Inc., a nanotechnology company, and was most recently the President and Chief Executive
179
Officer of Epic Sciences, Inc., a medical diagnostics company, from August 2013 through April 2019. Dr. Prahalad has two decades of experience in the technology and life science industries. From 2007 through 2013, Dr. Prahalad served in multiple roles at Life Technologies, including as Vice President of Corporate Strategy. Before Life Technologies, Dr. Prahalad was Vice President of Business Development at Sequenom, Inc., a biotechnology company. Dr. Prahalad received a PhD in biochemistry and molecular pharmacology as well as an MS in medical sciences from Harvard University. He also holds a BS in Cellular and Molecular Biology and Economics from the University of Michigan. Mr. Prahalad served on the board of managers of MLSH 1 and will continue to serve until he is appointed as director in connection with the closing of the offering. We determined Dr. Prahalad’s extensive experience in the technology and life sciences industry, in addition to his medical expertise and experience on boards of directors, qualifies him to serve as a director on the Board of Directors.
Family Relationships
There are no family relationships between any of our executive officers, directors or director nominees.
Corporate Governance
Board Composition and Director Independence
Our business and affairs are managed under the direction of our Board. Following completion of this offering, our Board will be composed of 11 directors. Our certificate of incorporation will provide that the authorized number of directors may be changed only by resolution of our Board. In addition, the Director Nomination Agreement will prohibit us from increasing or decreasing the size of our Board without the prior written consent of GTCR. Our certificate of incorporation will also provide that our Board will be divided into three classes of directors, with the classes as nearly equal in number as possible. Subject to any earlier resignation or removal in accordance with the terms of our certificate of incorporation and bylaws, our Class I directors will be Messrs. Hull, Daverman and Mihas and Ms. Gray and will serve until the first annual meeting of shareholders following the completion of this offering, our Class II directors will be Messrs. Cunningham and Hance and Drs. Hopfield and Prahalad and will serve until the second annual meeting of shareholders following the completion of this offering and our Class III directors will be Messrs. Lucier and Marker and Ms. Ashkenazi and will serve until the third annual meeting of shareholders following the completion of this offering. Upon completion of this offering, we expect that each of our directors will serve in the classes as indicated above. This classification of our Board could have the effect of increasing the length of time necessary to change the composition of a majority of the Board. In general, at least two annual meetings of shareholders will be necessary for shareholders to effect a change in a majority of members of the Board. In addition, our certificate of incorporation will provide that our directors may be removed with or without cause by the affirmative vote of at least a majority of the voting power of our outstanding shares of stock entitled to vote thereon, voting together as a single class for so long as GTCR beneficially owns 40% or more, in the aggregate, of the total number of shares of our common stock then outstanding. If GTCR’s aggregate beneficial ownership falls below 40% of the total number of shares of our common stock outstanding, then our directors may be removed only for cause upon the affirmative vote of at least 66 2/3% of the voting power of our outstanding shares of stock entitled to vote thereon.
In addition, at any time when GTCR has the right to designate at least one nominee for election to our Board, GTCR will also have the right to have one of its nominated directors hold one seat on each Board committee, subject to satisfying any applicable stock exchange rules or regulations regarding the independence of Board committee members. The listing standards of NASDAQ require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act.
Our Board has also determined that Messrs. Hance and Lucier, Ms. Ashkenazi, Ms. Gray and Drs. Hopfield and Prahalad meet the requirements to be independent directors. In making this determination, our Board
180
considered the relationships that each such non-employee director has with Maravai and all other facts and circumstances that our Board deemed relevant in determining their independence, including beneficial ownership of our common stock.
Controlled Company Status
After completion of this offering, GTCR will continue to control a majority of the voting power in us. As a result, we will be a “controlled company.” Under NASDAQ rules, a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including the requirements that, within one year of the date of the listing of our common stock:
| • | we have a board of directors that is composed of a majority of “independent directors,” as defined under the rules of such exchange; |
| • | we have a compensation committee that is composed entirely of independent directors; and |
| • | we have a nominating and corporate governance committee that is composed entirely of independent directors. |
Following this offering, we intend to rely on this exemption. As a result, we may not have a majority of independent directors on our Board. In addition, our Compensation Committee and our Nominating and Corporate Governance Committee may not consist entirely of independent directors or be subject to annual performance evaluations. Accordingly, you may not have the same protections afforded to shareholders of companies that are subject to all of the NASDAQ corporate governance requirements.
Board Committees
Upon completion of this offering, our Board will have an Audit Committee and a Compensation and Nominating Committee. The composition, duties and responsibilities of these committees are as set forth below. In the future, our Board may establish other committees, as it deems appropriate, to assist it with its responsibilities.
| Board Member |
Audit Committee | Compensation and Nominating Committee |
||||||
| Carl Hull |
||||||||
| Anat Ashkenazi* |
X | |||||||
| Sean Cunningham* |
||||||||
| Benjamin Daverman* |
X | |||||||
| Susannah Gray* |
X (Chair) | |||||||
| Robert B. Hance* |
||||||||
| Jessica Hopfield* |
X | X | ||||||
| Gregory T. Lucier* |
||||||||
| Luke Marker* |
||||||||
| Constantine Mihas* |
X (Chair) | |||||||
| Murali K. Prahalad* |
||||||||
| * | Denotes director nominee |
Audit Committee
Following this offering, our Audit Committee will be composed of Dr. Hopfield, Ms. Gray and Ms. Ashkenazi, with Ms. Gray serving as chairman of the committee. We intend to comply with the audit
181
committee requirements of the SEC and NASDAQ, which require that the Audit Committee be composed of at least one independent director at the closing of this offering, a majority of independent directors within 90 days following this offering and all independent directors within one year following this offering. We anticipate that, prior to the completion of this offering, our Board will determine that Dr. Hopfield, Ms. Gray and Ms. Ashkenazi meet the independence requirements of Rule 10A-3 under the Exchange Act and the applicable listing standards of NASDAQ. Our Board has determined that Ms. Gray and Ms. Ashkenazi are “audit committee financial experts” within the meaning of SEC regulations and applicable listing standards of NASDAQ. The Audit Committee’s responsibilities upon completion of this offering will include:
| • | appointing, approving the compensation of, and assessing the qualifications, performance and independence of our independent registered public accounting firm; |
| • | pre-approving audit and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
| • | discusses on a periodic basis, or as appropriate, with management, our policies, programs and controls with respect to risk assessment and risk management; |
| • | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us; |
| • | is responsible for reviewing our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; |
| • | monitors the rotation of partners of the independent registered public accounting firm on our engagement team in accordance with requirements established by the SEC; |
| • | reviews management’s report on its assessment of the effectiveness of internal control over financial reporting and any changes thereto; |
| • | reviewing the adequacy of our internal control over financial reporting; |
| • | establishing policies and procedures for the receipt, retention, follow-up and resolution of accounting, internal controls or auditing matters, complaints and concerns; |
| • | recommending, based upon the Audit Committee’s review and discussions with management and the independent registered public accounting firm, whether our audited financial statements shall be included in our Annual Report on Form 10-K; |
| • | monitoring our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters; |
| • | preparing the Audit Committee report required by the rules of the SEC to be included in our annual proxy statement; |
| • | annually reviews and assesses treasury functions including cash management process; |
| • | investigates any matters received, and reports to the Board periodically, with respect to ethics issues, complaints and associated investigations; |
| • | reviews the audit committee charter and the committee’s performance at least annually; |
| • | consults with management to establish procedures and internal controls relating to cybersecurity; |
| • | reviewing all related party transactions for potential conflict of interest situations and approving all such transactions; and |
| • | reviewing and discussing with management our earnings releases and scripts. |
182
Compensation and Nominating Committee
Following this offering, our Compensation and Nominating Committee will be composed of Messrs. Mihas and Daverman and Dr. Hopfield, with Mr. Mihas serving as chairman of the committee. The Compensation and Nominating Committee’s responsibilities upon completion of this offering will include:
| • | annually reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer; |
| • | evaluating the performance of our chief executive officer in light of such corporate goals and objectives and determining and approving the compensation of our chief executive officer; |
| • | reviewing and approving the compensation of our other executive officers; |
| • | appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
| • | conducting the independence assessment outlined in NASDAQ rules with respect to any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
| • | annually reviewing and reassessing the adequacy of the committee charter in its compliance with the listing requirements of NASDAQ; |
| • | reviewing and establishing our overall management compensation, philosophy and policy; |
| • | overseeing and administering our compensation and similar plans; |
| • | reviewing and making recommendations to our Board with respect to director compensation; |
| • | reviewing and discussing with management the compensation discussion and analysis to be included in our annual proxy statement or Annual Report on Form 10-K; |
| • | developing and recommending to our Board criteria for board and committee membership; |
| • | subject to the rights of GTCR under the Director Nomination Agreement as described in “Certain Relationships and Related Party Transactions—Director Nomination Agreement,” identifying and recommending to our Board the persons to be nominated for election as directors and to each of our Board’s committees; |
| • | developing and recommending to our Board best practices and corporate governance principles; |
| • | developing and recommending to our Board a set of corporate governance guidelines; and |
| • | reviewing and recommending to our Board the functions, duties and compositions of the committees of our Board. |
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past fiscal year has served, as a member of the Board or compensation committee of any entity that has one or more executive officers serving on our Board or Compensation and Nominating Committee.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Upon the closing of this offering, our code of business conduct and ethics will be available on our website. We intend to disclose any amendments to the code, or any waivers of its requirements, on our website.
183
Unless we state otherwise or the context otherwise requires, in this Executive Compensation section the terms “Maravai LifeSciences,” “we,” “us,” “our” and the “Company” refer to Topco LLC, for the period up to this offering, and for all periods following this offering, to Maravai LifeSciences Holdings, Inc.
We are currently considered an “emerging growth company” within the meaning of the Securities Act for purposes of the SEC’s executive compensation disclosure rules. Accordingly, we are required to provide a Summary Compensation Table and an Outstanding Equity Awards at Fiscal Year-End Table, as well as limited narrative disclosures regarding executive compensation for our last completed fiscal year. Further, our reporting obligations extend only to the following “Named Executive Officers,” who are the individuals who served as our principal executive officer during and the next two most highly compensated executive officers at the end of the fiscal year ended December 31, 2019. For the fiscal year ended December 31, 2019, our Named Executive Officers and their principal positions were as follows:
| • | Carl Hull, Chief Executive Officer of the Company; |
| • | Kevin Herde, Chief Financial Officer of the Company; and |
| • | Brian Neel, Chief Operating Officer, Nucleic Acid Production. |
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt in the future may differ materially from the currently planned programs summarized in this discussion.
Summary Compensation Table
| Name and principal position |
Year | Salary ($) |
Option awards ($)(1) |
Non-equity incentive plan compensation ($)(2) |
All other compensation ($)(3) |
Total ($) |
||||||||||||||||||
| Carl Hull, Chief Executive Officer |
2019 | 485,417 | 1,743,000 | 600,000 | — | 2,828,417 | ||||||||||||||||||
| Kevin Herde, Chief Financial Officer |
2019 | 364,160 | 535,000 | 165,063 | 8,400 | 1,072,623 | ||||||||||||||||||
| Brian Neel, Chief Operating Officer, Nucleic Acid Production |
2019 | 314,673 | 428,000 | 142,436 | 8,400 | 893,509 | ||||||||||||||||||
| (1) | The amounts reported in the Option Awards column represent the grant date fair value of the incentive units in MLSH 1 granted to the Named Executive Officers as computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718. The assumptions used in calculating the grant date fair value of the incentive units in MLSH 1 reported in the Option Awards column are set forth in Note 8 to the consolidated financial statements included elsewhere in this prospectus. The amounts reported in this column reflect the accounting cost for these incentive units and do not correspond to the actual economic value that may be received by the Named Executive Officers for the incentive units. See “Narrative Disclosure to Summary Compensation Table—Equity Incentives” below for additional details. |
| (2) | The amounts reported in the Non-Equity Incentive Plan Compensation column reflect bonuses paid to the Named Executive Officers under the Bonus Plan (as defined below) with respect to the fiscal year ended December 31, 2019. Please see the section entitled “Narrative Disclosure to Summary Compensation Table—Employment Agreements” below for additional details. |
| (3) | The amounts reported in the All Other Compensation column reflect 401(k) plan matching contributions made on behalf of the Named Executive Officers during the fiscal year ended December 31, 2019. See below under “Additional Narrative Disclosure—Retirement Benefits” for additional information regarding 401(k) plan contributions. |
184
Narrative Disclosure to Summary Compensation Table
Employment Agreements
We have entered into employment agreements (styled as senior management agreements) with each of our Named Executive Officers that provide for annual base salary, target bonus opportunity, a purchase of capital units and an initial grant of incentive units in MLSH 1, paid vacation, reimbursement of reasonable business expenses and eligibility to participate in our benefit plans generally.
Messrs. Hull’s, Herde’s and Neel’s annualized base salaries at the end of the 2019 fiscal year were $500,000, $367,787 and $317,369, respectively, and their target annual bonuses were 100%, 40% and 40% of base salary. For the 2019 fiscal year, Messrs. Hull, Herde and Neel received bonus payments of $600,000, $165,063 and $142,435, respectively, based in part on pre-established company performance metrics and based in part on individual achievement. The pre-established company performance metrics for the 2019 fiscal year consisted of adjusted revenue (weighted 30%), Adjusted EBITDA (weighted 50%), and achievement of corporate initiatives (weighted 20%). For the 2019 fiscal year, we achieved the pre-established company performance metrics at 102% of target. The company performance metric score was then adjusted based on individual achievement to yield total achievement scores, which corresponded to payouts of 120% of target for Mr. Hull and 112.2% of target for Messrs. Herde and Neel.
The employment agreements also provide for certain severance benefits upon a resignation by the applicable Named Executive Officer for “good reason” or upon a termination by MLSH 1 without “cause.” Please see the section entitled “Additional Narrative Disclosure—Potential Payments Upon Termination or Change in Control” below for more details regarding the severance benefits provided to our Named Executive Officers under the employment agreements.
Equity Incentives
We have historically offered equity incentives to our Named Executive Officers through grants of incentive units in MLSH 1. Certain of these incentive unit awards are subject to time-based vesting requirements and are subject to accelerated vesting upon the occurrence of certain terminations of employment and certain change-in-control events, and the remaining incentive unit awards are subject to market and performance-based vesting requirements and terminate if such performance-based vesting requirements are not met upon certain change-in-control events. We do not anticipate that the consummation of this offering or any of the related transactions will trigger accelerated vesting of any of the incentive units in MLSH 1 that are subject to time-based vesting requirements, but we expect the vesting of the incentive units subject to performance-based vesting requirements to be accelerated in connection with this offering. See below under “—Additional Narrative Disclosure—Potential Payments Upon Termination or Change in Control” for additional information regarding the circumstances that could result in accelerated vesting of these awards and under “—Actions Taken in 2020 or in Connection with this Offering” for additional information regarding the expected acceleration of the units subject to performance-based vesting.
185
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes, for each of the Named Executive Officers, the number of incentive units in MLSH 1 held as of December 31, 2019.
| Option Awards(1) | ||||||||||||
| Name |
Number of securities underlying unexercised options (#) exercisable |
Number of securities underlying unexercised options (#) unexercisable |
Option exercise price ($)(8) |
Option expiration date(8) | ||||||||
| Carl Hull |
272,000 | 203,000 | (2) | N/A | N/A | |||||||
| 14,000 | 86,000 | (3) | N/A | N/A | ||||||||
| Kevin Herde |
30,000 | 70,000 | (4) | N/A | N/A | |||||||
| — | 25,000 | (5) | N/A | N/A | ||||||||
| Brian Neel |
14,000 | 51,000 | (6) | N/A | N/A | |||||||
| — | 20,000 | (7) | N/A | N/A | ||||||||
| (1) | This table reflects information regarding incentive units in MLSH 1 granted to our Named Executive Officers that were outstanding as of December 31, 2019. For more information on these incentive units, see “Narrative Disclosure to Summary Compensation Table—Equity Incentives” above. |
| (2) | Under the terms of the applicable incentive unit award documentation, (i) 75,000 of these incentive units will vest upon achievement of a certain multiple of pre-public offering investor proceeds over initial investments, so long as Mr. Hull remains employed through the date of such achievement, but these units are expected to become vested in connection with this offering; and (ii) 64,000 of these incentive units will vest on April 5, 2021, so long as Mr. Hull remains employed through such date, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (3) | Under the terms of the applicable incentive unit award documentation, (i) 30,000 of these incentive units will vest upon achievement of a certain multiple of investor proceeds over initial investments, so long as Mr. Hull remains employed through the date of such achievement, but these units are expected to become vested in connection with this offering; and (ii) 56,000 of these incentive units will vest in equal installments on June 20 of each of 2021, 2022, 2023 and 2024, so long as Mr. Hull remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (4) | Under the terms of the applicable incentive unit award documentation, (i) 25,000 of these incentive units will vest upon achievement of a certain multiple of investor proceeds over initial investments, so long as Mr. Herde remains employed through the date of such achievement, but these units are expected to become vested in connection with this offering; and (ii) 30,000 of these incentive units will vest in equal installments on May 30 of each of 2021 and 2022, so long as Mr. Herde remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (5) | Under the terms of the applicable incentive unit award documentation, these incentive units will vest in equal installments on December 13 of each of 2020, 2021, 2022, 2023 and 2024, so long as Mr. Herde remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (6) | Under the terms of the applicable incentive unit award documentation, (i) 30,000 of these incentive units will vest upon achievement of a certain multiple of investor proceeds over initial investments, so long as Mr. Neel remains employed through the date of such achievement, but these units are expected to become vested in connection with this offering; (ii) 7,000 of these incentive units vested on October 16, 2020; and (iii) 21,000 of these incentive units will vest in equal installments on October 16 of each of 2021 and 2022, so long as Mr. Neel remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
186
| (7) | Under the terms of the applicable incentive unit award documentation, these incentive units will vest in equal installments on December 13 of each of 2020, 2021, 2022, 2023 and 2024, so long as Mr. Neel remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (8) | These equity awards are not traditional options and, therefore, there is no exercise price or option expiration date associated with them. |
Additional Narrative Disclosure
Retirement Benefits
We do not have a defined benefit pension plan or nonqualified deferred compensation plan. We currently maintain a retirement plan intended to provide benefits under Section 401(k) of the Code, pursuant to which employees, including the Named Executive Officers, can make voluntary pre-tax contributions. We match 50% of elective deferrals up to 6% of elective deferrals for all participants. These matching contributions are vested or vest based on the participant’s length of service with us, becoming fully vested on the fourth anniversary of the participant’s date of hire. All contributions under the plan are subject to certain annual dollar limitations, which are periodically adjusted for changes in the cost of living.
Potential Payments Upon Termination or Change in Control
A Named Executive Officer’s outstanding incentive units in MLSH 1 that vest based on time will become 100% vested upon a “sale” of MLSH 1, which is generally the sale of (i) MLSH 1’s equity securities pursuant to which an independent third party or parties acquires a majority of the equity securities or voting power to elect a majority of the board of directors of MLSH 1 or (ii) all or substantially all of MLSH 1’s assets on a consolidated basis. We do not anticipate that the consummation of this offering or any of the related transactions will constitute a “sale” of MLSH 1 for this purpose.
Our Named Executive Officers’ employment agreements provide that upon a termination by us for any reason other than for “cause” or upon a resignation by such Named Executive Officer for “good reason,” each as defined therein, subject to the execution and delivery of a fully effective release of claims in favor of the Company and continued compliance with applicable restrictive covenants, Mr. Hull will receive salary continuation payments and COBRA premium reimbursement for 12 months, Mr. Herde will receive salary continuation payments and COBRA premium reimbursement for 6 months, and Mr. Neel will receive salary continuation payments and COBRA premium reimbursement for 3 months. The employment agreements also contain certain restrictive covenants, including provisions that create restrictions, with certain limitations, on our Named Executive Officers competing with MLSH 1 and its subsidiaries during the term of the Named Executive Officer’s employment with the Company (and, if the Named Executive Officer’s equity is repurchased, for the one-year period following termination of employment) and soliciting any customers or other business relations or soliciting or hiring employees of MLSH 1 and its subsidiaries, in each case, during the term of the Named Executive Officer’s employment with the Company and for the one-year period following termination of employment.
Actions Taken in 2020 or in Connection with This Offering
Acceleration of Incentive Units Subject to Performance-Based Vesting
In connection with this offering, we expect the vesting of incentive units held by our Named Executive Officers that are subject to performance-based vesting requirements to be accelerated. See the “Outstanding Equity Awards at Fiscal Year-End Table” above for additional details regarding the incentive units subject to performance-based vesting that are held by our Named Executive Officers.
187
New Employment Agreements
It is anticipated that each of the Named Executive Officers will enter into a new employment agreement, which will be effective upon the closing of this offering (the “New Employment Agreements”) and will supersede each Named Executive Officer’s senior management agreement. The material terms and conditions set forth in each Named Executive Officer’s New Employment Agreement are anticipated to be substantially similar to those set forth in each Named Executive Officer’s existing senior management agreement and described herein. However, the base salary for Mr. Herde and Mr. Neel will be increased to $382,499 and $333,237, respectively.
Stock Option Grants
We expect to grant certain of our employees, including our Named Executive Officers, stock options under the 2020 Plan with respect to an aggregate of up to approximately 1,584,400 shares of the Company’s common stock. These stock option awards will vest over four years with 25% vesting on the first anniversary of the grant date and the remaining award vesting monthly over the three-year period thereafter, subject to the recipient’s continued employment through each vesting date.
2020 Employee Stock Purchase Plan
In order to incentivize our employees following the completion of this offering, we anticipate that our Board will adopt the ESPP, the material terms of which are summarized below, prior to the completion of this offering. This summary is not a complete description of all of the provisions of the ESPP and is qualified in its entirety by reference to the ESPP, a copy of which will be filed as an exhibit to the registration statement of which this prospectus forms a part.
While we continue to maintain an “Up-C” structure, with many of our employees employed by subsidiaries of Topco LLC, we are not eligible to satisfy certain of the rules for maintaining a tax-qualified “employee stock purchase plan” under Section 423 of the Code, which rules only permit participation in such a plan by employees of a “subsidiary corporation” as defined in Section 424(f) of the Code. Thus, when the ESPP becomes effective, it will be operated as a nonqualified plan. However, other than with respect to certain eligibility limitations (described below), which will be operative only while we are ineligible to maintain a qualified plan, the ESPP is designed to comply with the requirements of Section 423 of the Code. Thus, it is intended that if and when the ESPP can be operated as a tax-qualified plan, as determined by the compensation committee, the ESPP will be a qualified “employee stock purchase plan” under Section 423 of the Code, and will be operated and administered with that intent thereafter.
Shares Available for Awards; Administration
A total of 10,313,540 shares of our Class A common stock will initially be reserved for issuance under the ESPP. In addition, the number of shares available for issuance under the ESPP will be increased annually on January 1 of each calendar year beginning in 2021 and ending in and including 2030, by an amount equal to the lesser of (A) 1.25% of the shares outstanding on the final day of the immediately preceding calendar year and (B) such smaller number of shares as is determined by our Board. In no event will more than 42,543,353 shares of our Class A common stock be available for issuance under the ESPP. Our Board or a committee of our Board will administer and will have authority to interpret the terms of the ESPP and determine eligibility of participants. We expect that the compensation committee will be the initial administrator of the ESPP.
Eligibility
We expect that all of our employees and employees of any designated subsidiary, as defined in the ESPP, will be eligible to participate in the ESPP, other than those employees whose customary employment is for thirty
188
hours or less per week. However, an employee may not be granted rights to purchase stock under our ESPP if the employee, immediately after the grant, would own (directly or through attribution) stock possessing 5% or more of the total combined voting power of all classes of our stock.
Grant of Rights
Stock will be offered under the ESPP during offering periods. Each offering period other than the initial offering period will be approximately 24 months in length commencing on each May 1 and November 1 during the term of the ESPP. The plan administrator may, at its discretion, choose a different length of the offering period not to exceed 27 months. Employee payroll deductions will be used to purchase shares on each purchase date during an offering period. The purchase date for each offering period will be the final trading day in the offering period. The plan administrator may, in its discretion, modify the terms of future offering periods.
The ESPP permits participants to purchase Class A common stock through payroll deductions of up to 15% of their eligible compensation. The maximum number of shares that may be purchased by a participant during any offering period will be 2,000 shares. In addition, no employee will be permitted to accrue the right to purchase stock at a rate in excess of $25,000 worth of shares during any calendar year during which such a purchase right is outstanding (based on the fair market value per share of our Class A common stock as of the first day of the offering period).
On the first trading day of each offering period, each participant will automatically be granted an option to purchase shares of our Class A common stock. The option will expire at the end of the applicable offering period, and will be exercised at that time to the extent of the payroll deductions accumulated during the offering period. The purchase price of the shares, in the absence of a contrary designation, will be 85% of the lower of the fair market value of our Class A common stock on the first trading day of the offering period or on the purchase date. Participants may voluntarily end their participation in the ESPP at any time during a specified period prior to the end of the applicable offering period, and will be paid their accrued payroll deductions that have not yet been used to purchase shares of Class A common stock. Participation ends automatically upon a participant’s termination of employment.
A participant may not transfer rights granted under the ESPP other than by will or the laws of descent and distribution, and rights granted under the ESPP are generally exercisable only by the participant.
Certain Transactions
In the event of certain transactions or events affecting our Class A common stock, the plan administrator will make equitable adjustments to the ESPP and outstanding rights. In the event of certain unusual or non-recurring events or transactions, including a change in control, the plan administrator may provide for (1) either the replacement of outstanding rights with other rights or property or termination of outstanding rights in exchange for cash, (2) the assumption or substitution of outstanding rights by the successor or survivor corporation or parent or subsidiary thereof, if any, (3) an adjustment to the number and type of shares of stock subject to outstanding rights, (4) the use of participants’ accumulated payroll deductions to purchase stock on a new purchase date prior to the next scheduled purchase date and termination of any rights under ongoing offering periods or (5) the termination of all outstanding rights.
Plan Amendment
The plan administrator may amend, suspend or terminate the ESPP at any time. However, stockholder approval will be obtained for any amendment that increases the aggregate number or changes the type of shares that may be sold pursuant to rights under the ESPP or changes the corporations or classes of corporations whose employees are eligible to participate in the ESPP.
189
2020 Omnibus Incentive Plan
In order to incentivize our employees following the completion of this offering, we anticipate that our Board will adopt the 2020 Plan, for employees, consultants and directors prior to the completion of this offering. This summary is not a complete description of all of the provisions of the 2020 Plan and is qualified in its entirety by reference to the 2020 plan, a copy of which will be filed as an exhibit to the registration statement of which this prospectus forms a part. Our Named Executive Officers will be eligible to participate in the 2020 Plan, which we expect will become effective upon the consummation of this offering. We anticipate that the 2020 Plan will provide for the grant of options, stock appreciation rights, restricted stock, restricted stock units, stock awards, dividend equivalents, other stock-based awards, cash awards and substitute awards intended to align the interests of service providers, including our Named Executive Officers, with those of our shareholders.
Securities to be Offered
Subject to adjustment in the event of certain transactions or changes of capitalization in accordance with the 2020 Plan, a total of 25,783,851 shares of Class A common stock will initially be reserved for issuance pursuant to awards under the 2020 Plan. No more than 38,160,100 shares of Class A common stock may be issued under the 2020 Plan upon the exercise of incentive options. Shares of Class A common stock subject to an award that expires or is canceled, forfeited, exchanged, settled in cash or otherwise terminated without delivery of shares and shares withheld to pay the exercise price of, or to satisfy the withholding obligations with respect to, an award will again be available for delivery pursuant to other awards under the 2020 Plan.
The total number of shares reserved for issuance under the 2020 Plan will be increased on January 1 of each of the first 10 calendar years during the term of the 2020 Plan, by the lesser of (i) 4% of the total number of shares of Class A common stock outstanding on each December 31 immediately prior to the date of increase or (ii) such number of shares of Class A common stock determined by our Board or compensation committee.
Administration
The 2020 Plan will be administered by our Board, except to the extent our Board elects a committee of directors to administer the 2020 Plan (as applicable, the “Administrator”). The Administrator has broad discretion to administer the 2020 Plan, including the power to determine the eligible individuals to whom awards will be granted, the number and type of awards to be granted and the terms and conditions of awards. The Administrator may also accelerate the vesting or exercise of any award and make all other determinations and to take all other actions necessary or advisable for the administration of the 2020 Plan. To the extent the Administrator is not our Board, our Board will retain the authority to take all actions permitted by the Administrator under the 2020 Plan.
Eligibility
Our employees, consultants and non-employee directors, and employees, consultants and non-employee directors of our affiliates, will be eligible to receive awards under the 2020 Plan.
Non-Employee Director Compensation Limits
Under the 2020 Plan, in a single calendar year, a non-employee director may not be granted awards for such individual’s service on our Board having a value in excess of $500,000 (calculating the value of any awards based on the grant date fair value for financial reporting purposes), provided that, for any fiscal year in which a non-employee director (i) first commences service on the Board, (ii) serves on a special committee of the Board, or (iii) serves as lead director or chairman of the Board, such limit shall be $750,000. Additional awards may be granted for any calendar year in which a non-employee director first becomes a director, serves on a special committee of our Board, or serves as lead director. This limit does not apply to cash fees or awards granted in lieu of cash fees.
190
Types of Awards
Options. We may grant options to eligible persons, except that incentive options may only be granted to persons who are our employees or employees of one of our subsidiaries, in accordance with Section 422 of the Code. The exercise price of an option generally cannot be less than 100% of the fair market value of a share of Class A common stock on the date on which the option is granted and the option must not be exercisable for longer than ten years following the date of grant. In the case of an incentive option granted to an individual who owns (or is deemed to own) at least 10% of the total combined voting power of all classes of our equity securities, the exercise price of the option must be at least 110% of the fair market value of a share of Class A common stock on the date of grant and the option must not be exercisable more than five years from the date of grant.
SARs. A stock appreciation right (“SAR”) is the right to receive an amount equal to the excess of the fair market value of one share of Class A common stock on the date of exercise over the grant price of the SAR. The grant price of a SAR generally cannot be less than 100% of the fair market value of a share of Class A common stock on the date on which the SAR is granted. The term of a SAR may not exceed ten years. SARs may be granted in connection with, or independent of, other awards. The Administrator will have the discretion to determine other terms and conditions of an SAR award.
Restricted Share Awards. A restricted share award is a grant of shares of Class A common stock subject to the restrictions on transferability and risk of forfeiture imposed by the Administrator. Unless otherwise determined by the Administrator and specified in the applicable award agreement, the holder of a restricted share award will have rights as a shareholder, including the right to vote the shares of Class A common stock subject to the restricted share award or to receive dividends on the shares of Class A common stock subject to the restricted share award during the restriction period. In the discretion of the Administrator, dividends distributed prior to vesting may be subject to the same restrictions and risk of forfeiture as the restricted shares with respect to which the distribution was made.
Restricted Stock Units (“RSU”). An RSU is a right to receive cash, shares of Class A common stock or a combination of cash and shares of Class A common stock at the end of a specified period equal to the fair market value of one share of Class A common stock on the date of vesting. RSUs may be subject to the restrictions, including a risk of forfeiture, imposed by the Administrator.
Share awards. A share award is a transfer of unrestricted shares of Class A common stock on terms and conditions, if any, determined by the Administrator.
Dividend Equivalents. Dividend equivalents entitle a participant to receive cash, shares of Class A common stock, other awards or other property equal in value to dividends or other distributions paid with respect to a specified number of shares of Class A common stock. Dividend equivalents may be granted on a free-standing basis or in connection with another award (other than a restricted share award or a share award).
Other Share-Based Awards. Other share-based awards are awards denominated or payable in, valued in whole or in part by reference to, or otherwise based on or related to, the value of our shares of Class A common stock.
Cash Awards. Cash awards may be granted on a free-standing basis or as an element of, a supplement to, or in lieu of any other award.
Substitute Awards. Awards may be granted in substitution or exchange for any other award granted under the 2020 Plan or under another equity incentive plan or any other right of an eligible person to receive payment from us. Awards may also be granted under the 2020 Plan in substitution for similar awards held for individuals who become participants as a result of a merger, consolidation or acquisition of another entity by or with us or one of our affiliates.
191
Certain Transactions
If any change is made to our capitalization, such as a share split, share combination, share dividend, exchange of shares or other recapitalization, merger or otherwise, which results in an increase or decrease in the number of outstanding shares of Class A common stock, appropriate adjustments will be made by the Administrator in the shares subject to an award under the 2020 Plan. The Administrator will also have the discretion to make certain adjustments to awards in the event of a change in control, such as accelerating the vesting or exercisability of awards, requiring the surrender of an award, with or without consideration, or making any other adjustment or modification to the award that the Administrator determines is appropriate in light of such transaction.
Clawback
All awards granted under the 2020 Plan will be subject to reduction, cancelation or recoupment under any written clawback policy that we may adopt and that we determine should apply to awards under the 2020 Plan.
Plan Amendment and Termination
Our Administrator may amend or terminate any award, award agreement or the 2020 Plan at any time; however, shareholder approval will be required for any amendment to the extent necessary to comply with applicable law or exchange listing standards. The Administrator will not have the authority, without the approval of shareholders, to amend any outstanding option or share appreciation right to reduce its exercise price per share. The 2020 Plan will remain in effect for a period of 10 years (unless earlier terminated by our Board).
Non-Employee Director Compensation
The following table presents the total compensation for each person who served as a non-employee member of our Board during 2019. Other than as set forth in the table and described more fully below, we did not pay any compensation, reimburse any expense of, make any equity awards or non-equity awards to, or pay any other compensation to any of, the other non-employee members of our Board in 2019.
| Name |
Fees earned or paid in cash ($)(1) |
Total ($) | ||||||
| Robert B. Hance(2) |
35,000 | 35,000 | ||||||
| Murali K. Prahalad(3) |
30,000 | 30,000 | ||||||
| (1) | Messrs. Hance and Prahalad are entitled to Board fees of $10,000 per meeting attended in person and $5,000 per meeting attended telephonically. In the 2019 fiscal year, Mr. Hance attended three meetings in person and one meeting telephonically and Mr. Prahalad attended two meetings in person and two meetings telephonically. |
| (2) | As of December 31, 2019, Mr. Hance held 4,000 vested incentive units and 6,000 unvested incentive units (2,000 of which vested on January 1, 2020, and the other 4,000 of which will vest in equal installments, subject to Mr. Hance’s continued service, on January 1 of 2021 and 2022) in MLSH 1. |
| (3) | As of December 31, 2019, Mr. Prahalad held 6,000 vested incentive units and 4,000 unvested incentive units (half of which vested on August 10, 2020, and the other half of which will vest, subject to Mr. Prahalad’s continued service, on August 10, 2021) in MLSH 1. |
Non-Employee Director Compensation Policy
Prior to this offering, we did not have a formal policy with respect to compensating our non-employee directors for service as directors. Each of Messrs. Hance, Lucier and Prahalad is subject to an investment and director compensation agreement with MLSH 1, pursuant to which they have been granted restricted incentive units in MLSH 1 and are entitled to director fees ($10,000 per meeting attended in person and $5,000 per meeting attended telephonically) and reimbursement of expenses incurred in connection with their service.
192
After the completion of this offering, our non-employee directors will be eligible to receive compensation for their service on our Board. We expect that, following this offering, our non-employee directors will receive the annual cash retainers listed below for their service on our Board. The non-employee directors who are employees of GTCR or its affiliates have agreed or are otherwise obligated to transfer all or a portion of the compensation they receive for their service as directors to GTCR or its affiliates. The retainers will be paid in four equal quarterly installments and prorated for any partial year of service on our board of directors.
| Position |
Retainer ($) | |||
| Board Member |
40,000 | |||
| Audit Committee: |
||||
| Chairperson |
20,000 | |||
| Committee Member |
10,000 | |||
| Compensation and Nominating Committee: |
||||
| Chairperson |
25,000 | |||
| Committee Member |
12,500 | |||
We expect that our non-employee directors who are not also our employees will receive restricted stock units with an aggregate grant date value of $160,000 and such award will vest on the first anniversary of the grant date. In addition, we expect that non-employee directors who are not also our employees or employees of GTCR or its affiliates will receive a one-time grant of restricted stock units with an aggregate grant date value of $320,000 and such award will vest annually over three years.
193
The following table sets forth information about the beneficial ownership of our Class A common stock and Class B common stock as of November 6, 2020, after giving effect to the Organizational Transactions:
| • | each person or group known to us who beneficially owns more than 5% of our Class A common stock or Class B common stock immediately prior to this offering; |
| • | each of our directors and director nominees; |
| • | each of our Named Executive Officers; and |
| • | all of our directors, director nominees and executive officers as a group. |
The numbers of shares of Class A common stock and Class B common stock (together with the same amount of LLC Units) beneficially owned and percentages of beneficial ownership before this offering that are set forth below are based on the number of shares and LLC Units to be issued and outstanding prior to this offering after giving effect to the Organizational Transactions. See “Organizational Structure.” The numbers of shares of Class A common stock and Class B common stock (together with the same amount of LLC Units) beneficially owned and percentages of beneficial ownership after the offering that are set forth below are based on 81,379,818 shares of Class A common stock to be issued and outstanding immediately after the offering, assuming no exercise by the underwriters of their option to purchase additional shares. This number excludes 176,458,692 shares of Class A common stock issuable in exchange for LLC Units and upon conversion of shares of our Class B common stock, each as described under “Organizational Structure” and “Certain Relationships and Related Party Transactions—Amended and Restated Operating Agreement.” If all outstanding LLC Units were exchanged and all outstanding shares of Class B common stock were converted, we would have 257,838,510 shares of Class A common stock outstanding immediately after this offering.
Concurrently with this offering, we will issue to the LLC Unitholders 176,458,692 shares of Class B common stock. The number of shares of Class B common stock will depend in part on the price at which shares of Class A common stock are sold in this offering after the offering. For purposes of the presentation of the total number of shares of Class B common stock beneficially owned, we have assumed that the shares of Class A common stock will be sold at $25.50 per share, which is the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus.
194
Unless otherwise noted below, the address for each beneficial owner listed on the table is 10770 Wateridge Circle Suite 200, San Diego, California, 92121. We have determined beneficial ownership in accordance with the rules of the SEC. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the tables below have sole voting and investment power with respect to all Class A common stock that they beneficially own, subject to applicable community property laws.
| Shares of Common Stock Beneficially Owned Prior to this Offering |
Shares of Common Stock Beneficially Owned After this Offering |
|||||||||||||||||||||||||||||||||||
| Name of Beneficial |
Shares of Class A Common Stock |
% of Class A Common Stock Outstanding |
Shares of Class B Common Stock |
% of Class B Common Stock Outstanding |
% of Combined Voting Power(1) |
Shares of Class A Common Stock |
Shares of Class B Common Stock |
% of Combined Voting Power Assuming the Underwriters’ Option Is Not Exercised(1) |
% of Combined Voting Power Assuming the Underwriters’ Option Is Exercised in Full(1) |
|||||||||||||||||||||||||||
| 5% Shareholders: |
||||||||||||||||||||||||||||||||||||
| GTCR(2) |
31,379,818 | 100 | % | 176,458,692 | 100 | % | 100 | % | 31,379,818 | 176,458,692 | 81 | % | 78 | % | ||||||||||||||||||||||
| Named Executive Officers, Directors and Director Nominees: |
||||||||||||||||||||||||||||||||||||
| Carl Hull |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Kevin Herde |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Brian Neel |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Anat Ashkenazi |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Sean Cunningham |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Benjamin Daverman |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Susannah Gray |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Robert B. Hance |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Jessica Hopfield |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Gregory T. Lucier |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Luke Marker |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Constantine Mihas |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Murali K. Prahalad |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| All executive officers, directors and director nominees as a group (16 individuals) |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| (1) | Each share of Class A common stock and Class B common stock entitles the registered holder thereof to one vote and each share on all matters presented to shareholders for a vote generally, including the election of directors. The Class A common stock and Class B common stock will vote as a single class on all matters except as required by law or the certificate of incorporation. |
| (2) | Represents 31,379,818 shares of Class A common stock held directly by MLSH 2 and 176,458,692 shares of Class B common stock held directly by MLSH 1. MLSH 1 and MLSH 2 are each managed by a board of managers. GTCR Fund XI/C LP controls the board of managers of MLSH 2. GTCR Fund XI/B LP and GTCR Co-Invest XI LP control the board of managers of MLSH 1. This number excludes 176,458,692 shares of Class A common stock issuable in exchange for LLC Units held by MLSH 1. These shares of Class A common stock represent approximately 80.6% of the shares of Class A common stock that would be outstanding immediately after this offering if all outstanding LLC Units were exchanged and all outstanding shares of Class B common stock were converted at that time. GTCR Partners XI/A&C LP is the general partner of GTCR Fund XI/C LP. GTCR Partners XI/B LP is the general partner of GTCR Fund XI/B LP. GTCR Investment XI LLC is the general partner of each of GTCR Co-Invest XI LP, GTCR Partners XI/A&C LP and GTCR Partners XI/B LP. GTCR Investment XI LLC is managed by a board of managers (the “GTCR Board of Managers”) consisting of Mark M. Anderson, Craig A. Bondy, Aaron D. Cohen, Sean L. Cunningham, Benjamin J. Daverman, David A. Donnini, Constantine S. Mihas and Collin E. Roche, and no single person has voting or dispositive authority over the Class A common stock or Class B common stock. Each of GTCR Partners XI/A&C LP, GTCR Investment XI LLC and the GTCR Board of Managers may be deemed to share beneficial ownership of the shares held of record by MLSH 2, each of GTCR Partners XI/B LP, GTCR Investment XI LLC and the GTCR Board of Managers may be deemed to share beneficial ownership of the shares held of record by MLSH 1 and each of the individual members of the GTCR Board of Managers disclaims beneficial ownership of the shares held of record by MLSH 1 and MLSH 2 except to the extent of his pecuniary interest therein. The address for each of MLSH 1, MLSH 2, GTCR Fund XI/C LP, GTCR Fund XI/B LP, GTCR Co-Invest XI LP, GTCR Partners XI/A&C LP, GTCR Partners XI/B LP and GTCR Investment XI LLC is 300 North LaSalle Street, Suite 5600, Chicago, IL, 60654, and their telephone number is (312) 382-2200. |
195
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Policies for Approval of Related Party Transactions
Prior to completion of this offering, we intend to adopt a policy with respect to the review, approval and ratification of related party transactions. Under the policy, our Audit Committee is responsible for reviewing and approving related party transactions. In the course of its review and approval of related party transactions, our Audit Committee will consider the relevant facts and circumstances to decide whether to approve such transactions. In particular, our policy requires our Audit Committee to consider, among other factors it deems appropriate:
| • | the related person’s relationship to us and interest in the transaction; |
| • | the material facts of the proposed transaction, including the proposed aggregate value of the transaction; |
| • | the impact on a director or a director nominee’s independence in the event the related person is a director or an immediate family member of the director or director nominee; |
| • | the benefits to us of the proposed transaction; |
| • | if applicable, the availability of other sources of comparable products or services; and |
| • | an assessment of whether the proposed transaction is on terms that are comparable to the terms available to an unrelated third party or to employees generally. |
The Audit Committee may only approve those transactions that are in, or are not inconsistent with, our best interests and those of our shareholders, as the Audit Committee determines in good faith.
Amended and Restated Operating Agreement
In connection with the completion of this offering, we will amend and restate Topco LLC’s existing operating agreement, which we refer to as the “LLC Operating Agreement.” The operations of Topco LLC and the rights and obligations of the LLC Unitholders will be set forth in the LLC Operating Agreement. See “Organizational Structure—Amended and Restated Operating Agreement of Topco LLC.”
Registration Rights Agreement
In connection with this offering, we intend to enter into a registration rights agreement with MLSH 1 and MLSH 2. MLSH 1 and MLSH 2 will be entitled to request that we register their shares of capital stock on a long-form or short-form registration statement on one or more occasions in the future, which registrations may be “shelf registrations.” MLSH 1 and MLSH 2 will be entitled to participate in certain of our registered offerings, subject to the restrictions in the registration rights agreement. We will pay expenses in connection with the exercise of these rights. The registration rights described in this paragraph apply to (1) shares of our Class A common stock held by MLSH 1 and MLSH 2 and their affiliates, and (2) any of our capital stock (or that of our subsidiaries) issued or issuable with respect to the Class A common stock described in clause (1) with respect to any dividend, distribution, recapitalization, reorganization, or certain other corporate transactions (“Registrable Securities”). These registration rights are also for the benefit of any subsequent holder of Registrable Securities; provided that any particular securities will cease to be Registrable Securities when they have been sold in a registered public offering, sold in compliance with Rule 144 of the Securities Act or repurchased by us or our subsidiaries. In addition, with the consent of the company and holders of a majority of Registrable Securities, certain Registrable Securities will cease to be Registrable Securities if they can be sold without limitation under Rule 144 of the Securities Act.
Tax Receivable Agreement
We intend to enter into a Tax Receivable Agreement with MLSH 1 and MLSH 2 that will provide for the payment from time to time by us to MLSH 1 and MLSH 2, collectively, of 85% of the amount of the benefits, if
196
any, that we realize or, under certain circumstances, are deemed to realize as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to this offering and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement. These payment obligations are obligations of Maravai LifeSciences Holdings, Inc. and not of Topco LLC. See “Organizational Structure—Tax Receivable Agreement.”
Director Nomination Agreement
In connection with this offering, we will enter into a Director Nomination Agreement with GTCR. The Director Nomination Agreement will provide GTCR the right to nominate to the Board a number of designees equal to at least: (i) 100% of the total number of directors comprising the Board, so long as GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 40% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering, (ii) 40% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 30% but less than 40% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering, (iii) 30% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 20% but less than 30% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering, (iv) 20% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 10% but less than 20% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering and (v) one director, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 5% of the total amount of shares of Class A common stock and Class B common stock it owns as of the date of this offering. In each case, GTCR’s nominees must comply with applicable law and stock exchange rules. In addition, GTCR shall be entitled to designate the replacement for any of its Board designees whose Board service terminates prior to the end of the director’s term, regardless of GTCR’s beneficial ownership at that time. GTCR shall also have the right to have its designees participate on committees of our Board proportionate to its voting power, subject to compliance with applicable law and stock exchange rules. The Director Nomination Agreement will also prohibit us from increasing or decreasing the size of our Board without the prior written consent of GTCR. This agreement will terminate at such time as GTCR beneficially owns less than 5% of the shares of Class A and Class B common stock it beneficially owns as of the date of this offering.
Indemnification of Officers and Directors
Upon completion of this offering, we intend to enter into indemnification agreements with each of our officers, directors and director nominees. The indemnification agreements will provide the officers and directors with contractual rights to indemnification, expense advancement and reimbursement, to the fullest extent permitted under Delaware law. Additionally, we may enter into indemnification agreements with any new directors or officers that may be broader in scope than the specific indemnification provisions contained in Delaware law. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our officers and directors pursuant to the foregoing agreements, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is therefore unenforceable.
Relationship with GTCR
We have utilized GTCR, who upon completion of this offering will control the vote of all matters submitted to a vote of our shareholders, for certain services pursuant to an advisory services agreement. Under this agreement, GTCR provides us with financial and management consulting services in the areas of corporate
197
strategy, budgeting for future corporate investments, acquisition and divestiture strategies and debt and equity financings. The advisory services agreement provides that we pay a $0.1 million quarterly management fee to GTCR for these services. We also reimburse GTCR for out-of-pocket expenses incurred while providing these services. The advisory services agreement also provides that we pay placement fees to GTCR of 1.0% of the gross amount of any debt or equity financings, including this offering. The advisory services agreement will terminate in connection with this offering.
We paid GTCR $0.6 million in the nine months ended September 30, 2020 and $0.6 million in each of the years ended December 31, 2019 and 2018. Following this offering, we may continue to engage GTCR from time to time, subject to compliance with our related party transactions policy.
During the year ended December 31, 2018, $52.0 million of capital distributions were made to the Class A unit holders of MLSC Holdings, LLC, including GTCR. No distributions were made to the Class A unit holders of MLSC Holdings, LLC during the year ended December 31, 2019. During the nine months ended September 30, 2020, $0.3 million of capital distributions were made to the Class A unit holders of MLSC Holdings, LLC, including GTCR. This distribution was treated as a return of capital per the terms of the MLSC LLC agreement, which will result in a reduction of future amounts that must be allocated to the Class A unit holders of MLSC, as controlling interest holders in MLSC, upon a liquidation of MLSC.
In October 2020, we paid GTCR a placement fee of $3.7 million in connection with our entry into the New Credit Agreement.
Lease Arrangements
Cygnus Technologies, a subsidiary of Topco LLC., has an ongoing lease agreement for facilities in Southport, NC with an entity controlled by a close relative of the president of Cygnus Technologies. The close relative was also previously an employee of Cygnus Technologies who terminated their employment during the year ended December 31, 2018. The president of Cygnus Technologies also personally financed a loan to this entity, which was used to acquire the property leased by Cygnus Technologies. The lease terms are considered to be consistent with market rates.
Cygnus Technologies paid $0.2 million of rent under this lease agreement for the nine months ended September 30, 2020 and each of the years ended December 31, 2019 and 2018.
Noncontrolling Interests
The noncontrolling interests in MLSC Holdings, LLC, the parent of Cygnus Technologies, represents equity interest that was retained by the unit holders of the MLSC Holdings, LLC entity prior to its acquisition by Maravai. The president of Cygnus Technologies and his affiliated entity are the holders of the noncontrolling interests.
Directed Share Program
At our request, the underwriters have reserved up to 1,725,000 shares of our Class A common stock, or 3% of the shares of our Class A common stock to be offered by this prospectus for sale, at the initial public offering price, for sale to certain individuals through a directed share program, including our directors, certain employees and certain other individuals identified by management.
198
DESCRIPTION OF CERTAIN INDEBTEDNESS
Set forth below is a summary of the terms of the agreements governing certain of our outstanding indebtedness. This summary is not a complete description of all of the terms of the agreements. The agreements setting forth the terms and conditions of certain of our outstanding indebtedness are filed as exhibits to the registration statement of which this prospectus forms a part.
New Credit Agreement
On October 19, 2020, Maravai Intermediate Holdings, LLC (“Intermediate”), a wholly-owned subsidiary of ours, along with its subsidiaries Vector Laboratories, TriLink BioTechnologies and Cygnus Technologies (together with Intermediate, the “Borrowers”), entered into a credit agreement (the “New Credit Agreement”) with Morgan Stanley Senior Funding, Inc. and certain other lenders, including affiliates of certain of the underwriters, providing for a $600.0 million term loan (the “New Term Loan”) and a $180.0 million revolving credit facility (the “New Revolving Credit Facility”). The entire amount of the New Term Loan was used on October 19, 2020 to refinance outstanding senior secured credit facilities, to make a distribution to MLSH 1, to repurchase minority interests at one of our subsidiaries, to pay transaction costs and expenses and for general corporate purposes. As of October 19, 2020, no amounts have been drawn against the New Revolving Credit Facility.
Interest Rates and Fees
Borrowings under the New Credit Agreement bear interest (a) initially, at the Borrowers’ option, either (i) at the Base Rate plus 3.25% per annum or (ii) the Adjusted Eurocurrency Rate plus 4.25% per annum and (b) after delivery of the compliance certificate for the fiscal quarter ending March 31, 2021, at the Borrowers’ option, either at (i) the Base Rate plus the applicable margin of 3.25% per annum with a stepdown to 3.00% based on the Borrowers’ consolidated first lien net leverage ratio or (ii) the Adjusted Eurocurrency Rate plus the margin of 4.25% per annum with a stepdown to 4.00% based on the Borrowers’ consolidated first lien net leverage ratio. The “Base Rate” is defined as the greatest of (i) the rate last quoted by The Wall Street Journal as the “Prime Rate” in the United States, (ii) the NYFRB Rate plus 0.50% per annum, (iii) the Adjusted Eurocurrency Rate for a one month interest period plus 1% per annum, (iv) solely with respect to the initial term loans, 2.00% per annum and (v) for any loans that are not initial term loans, 1.00% per annum. The “Adjusted Eurocurrency Rate” is defined as (a) with respect to the initial term loans, the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate (as such term is defined in the New Credit Agreement), and (ii) 1.00% and (b) with respect to the revolving loans, the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate (as such term is defined in the New Credit Agreement), and (ii) 0%. The “Eurocurrency Rate” is defined as LIBOR as displayed by Reuters (which if negative will be deemed to be 0%) or if LIBOR is unavailable, a rate based on historical LIBOR as determined by the administrative agent under the New Credit Agreement.
Accrued interest under the New Credit Agreement is payable (a) quarterly in arrears with respect to Base Rate loans, (b) at the end of each interest rate period (or at each three-month interval in the case of loans with interest periods greater than three months) with respect to Eurocurrency Rate loans, (c) on the date of any repayment or prepayment and (d) at maturity (whether by acceleration or otherwise). An annual commitment fee is applied to the daily unutilized amount under the New Revolving Credit Facility at 0.375% per annum, with one stepdown to 0.25% per annum based on the Borrowers’ consolidated first lien net leverage ratio.
Voluntary and Mandatory Prepayments
The New Term Loan contains prepayment provisions that allow the Borrowers, at their option, to prepay all or a portion of the principal amount at any time. Subject to certain exceptions and limitations and reinvestment rights, the Borrowers are required to repay borrowings under the New Term Loan and New Revolving Credit Facility with the proceeds of certain occurrences, such as the incurrence of debt and certain asset sales or
199
dispositions. The New Credit Agreement also requires mandatory prepayments to be calculated commencing with the fiscal year ending December 31, 2021 upon certain excess cash flow as defined in the terms of the agreement.
Final Maturity and Amortization
The New Term Loan becomes repayable in quarterly payments of $1.5 million beginning on March 31, 2021, with all remaining outstanding principal due at maturity on October 19, 2027. All outstanding amounts drawn under the New Revolving Credit Facility will become due at maturity on October 19, 2025.
Guarantees
Borrowings under the New Credit Agreement are unconditionally guaranteed by Topco LLC, together with the existing and future material domestic subsidiaries of Topco LLC (subject to certain exceptions), as specified in the respective guaranty agreements.
Security
Borrowings under the New Credit Agreement are secured by a first-priority lien and security interest in substantially all of the assets (subject to certain exceptions) of existing and future material domestic subsidiaries of Topco LLC that are loan parties.
Certain Covenants
The New Credit Agreement requires that, as of the end of any fiscal quarter where the aggregate amount of letter of credit obligations and revolving credit loans under the New Revolving Credit Facility outstanding as of such date (excluding cash collateralized letters of credit and letter of credit obligations in an aggregate amount not in excess of $5.0 million at any time outstanding and for the first four fiscal quarters ending after October 19, 2020, borrowings of revolving credit loans made on October 19, 2020) exceeds 35% of the aggregate amount of all revolving credit commitments under the New Revolving Credit Facility in effect as of such date, the Borrower’s consolidated first lien net leverage ratio shall not be greater than 8.00 to 1.00. The New Credit Agreement also contains negative and affirmative covenants, including covenants that restrict the ability of the Borrowers and their subsidiaries to, among other things, incur or prepay existing certain indebtedness, pay dividends or distributions, dispose of assets, engage in mergers and consolidations, make acquisitions or other investments and make changes in the nature of the business. The New Credit Agreement also requires Intermediate to provide audited consolidated financial statements to the lenders no later than 120 days after year-end.
Events of Default
The New Credit Agreement contains certain events of default, including, without limitation, nonpayment of principal, interest or other obligations, violation of the covenants, insolvency, court-ordered judgments and certain changes in control.
200
The following is a description of the material terms of our amended and restated certificate of incorporation and amended and restated bylaws, each as will be in effect at or prior to the consummation of this offering. The following description may not contain all of the information that is important to you. To understand the material terms of our Class A common stock, you should read our amended and restated certificate of incorporation and amended and restated bylaws, copies of which are or will be filed with the SEC as exhibits to the registration statement, of which this prospectus is a part.
General
At or prior to the consummation of this offering, we will file our amended and restated certificate of incorporation (our “certificate”), and we will adopt our amended and restated by-laws (our “bylaws”). Our certificate will authorize capital stock consisting of:
| • | shares of Class A common stock, par value $0.01 per share; |
| • | shares of Class B common stock, par value $0.01 per share; and |
| • | shares of preferred stock, with a par value per share that may be established by the Board in the applicable certificate of designations. |
We are selling 50,000,000 shares of Class A common stock in this offering (57,500,000 shares if the underwriters exercise in full their option to purchase additional shares). All shares of our Class A common stock outstanding upon consummation of this offering will be fully paid and non-assessable. We are issuing 176,458,692 shares of Class B common stock to MLSH 1 simultaneously with this offering (170,091,055 shares if the underwriters exercise in full their option to purchase additional shares of our Class A common stock). Upon completion of this offering, we expect to have 81,379,818 shares of Class A common stock outstanding (87,747,455 shares if the underwriters exercise in full their option to purchase additional shares) and 176,458,692 shares of Class B common stock outstanding (170,091,055 shares if the underwriters exercise in full their option to purchase additional shares).
The following summary describes the material provisions of our capital stock and is qualified in its entirety by reference to the certificate and our bylaws and to the applicable provisions of the DGCL. We urge you to read our certificate and our bylaws, which are included as exhibits to the registration statement of which this prospectus forms a part.
Certain provisions of our certificate and our bylaws summarized below may be deemed to have an anti-takeover effect and may delay or prevent a tender offer or takeover attempt that a shareholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares of common stock.
Class A Common Stock
Holders of shares of our Class A common stock are entitled to one vote for each share held of record on all matters submitted to a vote of shareholders. The holders of our Class A common stock do not have cumulative voting rights in the election of directors.
Holders of shares of our Class A common stock will vote together with holders of our Class B common stock as a single class on all matters presented to our shareholders for their vote or approval, except for certain amendments to our certificate of incorporation described below or as otherwise required by applicable law or the certificate.
Holders of shares of our Class A common stock are entitled to receive dividends when and if declared by our Board out of funds legally available therefor, subject to any statutory or contractual restrictions on the
201
payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock.
Upon our dissolution or liquidation or the sale of all or substantially all of our assets, after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of shares of our Class A common stock will be entitled to receive pro rata our remaining assets available for distribution.
Holders of shares of our Class A common stock do not have preemptive, subscription, redemption or conversion rights. There will be no redemption or sinking fund provisions applicable to the Class A common stock.
Class B Common Stock
Holders of shares of our Class B common stock are entitled to one vote for each share held of record on all matters submitted to a vote of shareholders. The holders of our Class B common stock do not have cumulative voting rights in the election of directors.
Holders of shares of our Class B common stock will vote together with holders of our Class A common stock as a single class on all matters presented to our shareholders for their vote or approval, except for certain amendments to our certificate of incorporation described below or as otherwise required by applicable law or the certificate.
Holders of our Class B common stock do not have any right to receive dividends or to receive a distribution upon dissolution or liquidation or the sale of all or substantially all of our assets. Additionally, holders of shares of our Class B common stock do not have preemptive, subscription, redemption or conversion rights. There will be no redemption or sinking fund provisions applicable to the Class B common stock. Any amendment of our certificate of incorporation that gives holders of our Class B common stock (1) any rights to receive dividends or any other kind of distribution, (2) any right to convert into or be exchanged for Class A common stock or (3) any other economic rights will require, in addition to shareholder approval, the affirmative vote of holders of our Class A common stock voting separately as a class.
Upon the consummation of this offering, MLSH 1 will own 100% of our outstanding Class B common stock.
Preferred Stock
Upon the consummation of this offering, we will have no shares of preferred stock outstanding.
Under the terms of our certificate that will become effective at or prior to the consummation of this offering, our Board is authorized to direct us to issue shares of preferred stock in one or more series without shareholder approval. Our Board has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our Board to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a shareholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock. Additionally, the issuance of preferred stock may adversely affect the holders of our Class A common stock by restricting dividends on the Class A common stock, diluting the voting power of the Class A common stock or subordinating the liquidation rights of the Class A common stock. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of our Class A common stock.
202
Forum Selection
Our certificate of incorporation will provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for any state court action for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our shareholders, (3) any action asserting a claim against Maravai LifeSciences Holdings, Inc. or any director or officer thereof arising pursuant to any provision of the DGCL or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware, our certificate of incorporation or our bylaws or (4) any other action asserting a claim against Maravai LifeSciences Holdings, Inc. or any director or officer thereof that is governed by the internal affairs doctrine; provided that, for the avoidance of doubt, the forum selection provision that identifies the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation, including any “derivative action,” will not apply to suits to enforce a duty or liability created by the Securities Act, the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our certificate of incorporation will also provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock will be deemed to have notice of and to have consented to the provisions of our certificate of incorporation described above. Although we believe these provisions benefit us by providing increased consistency in the application of Delaware law or the Securities Act, as applicable, for the specified types of actions and proceedings, the provisions may have the effect of discouraging lawsuits against us or our directors and officers. Alternatively, if a court were to find any of the forum selection provisions contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable, we may incur additional costs associated with having to litigate such action in other jurisdictions, which could have an adverse effect on our business, financial condition, results of operations, cash flows and prospects and result in a diversion of the time and resources of our employees, management and board of directors.
Anti-Takeover Provisions
Our certificate, bylaws and the DGCL contain provisions, which are summarized in the following paragraphs, that are intended to enhance the likelihood of continuity and stability in the composition of our Board. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile change of control and enhance the ability of our Board to maximize shareholder value in connection with any unsolicited offer to acquire us. However, these provisions may have an anti-takeover effect and may delay, deter or prevent a merger or acquisition of us by means of a tender offer, a proxy contest or other takeover attempt that a shareholder might consider in its best interest, including those attempts that might result in a premium over the prevailing market price for the shares of Class A common stock held by shareholders.
These provisions include:
Classified Board. Our certificate will provide that our Board will be divided into three classes of directors, with the classes as nearly equal in number as possible, and with the directors serving three-year terms. As a result, approximately one-third of our Board will be elected each year. The classification of the directors will have the effect of making it more difficult for shareholders to change the composition of our Board. Our certificate will also provide that, subject to any rights of holders of preferred stock to elect additional directors under specified circumstances, the number of directors will be fixed exclusively pursuant to a resolution adopted by our Board. Upon completion of this offering, we expect that our Board will have 11 members.
Shareholder Action by Written Consent. Our certificate will preclude shareholder action by written consent at any time when GTCR controls, in the aggregate, less than 35% in voting power of our outstanding common stock.
Special Meetings of Shareholders. Our certificate and bylaws will provide that, except as required by law, special meetings of our shareholders may be called at any time only by or at the direction of our Board or the
203
chairman of our Board; provided, however, at any time when GTCR controls, in the aggregate, at least 35% in voting power of our outstanding common stock, special meetings of our shareholders shall also be called by our Board or the chairman of our Board at the request of GTCR. Our bylaws will prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in control or management of us.
Advance Notice Procedures. Our bylaws will establish advance notice procedures for shareholder proposals and nomination of candidates for election as directors, other than nominations made by or at the direction of our Board or a committee of our Board, and provided, however, that at any time when GTCR controls, in the aggregate, at least 10% of the voting power of our outstanding common stock, such advance notice procedure will not apply to GTCR. Shareholders at an annual meeting will only be able to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our Board or by a shareholder who was a shareholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given our Secretary timely written notice, in proper form, of the shareholder’s intention to bring that business before the meeting. Although the bylaws will not give our Board the power to approve or disapprove shareholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting, the bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of us. These provisions do not apply to nominations by GTCR pursuant to the Director Nomination Agreement. See “Certain Relationships and Related Party Transactions—Director Nomination Agreement” for more details with respect to the Director Nomination Agreement.
Removal of Directors; Vacancies. Our certificate will provide that a director nominated by GTCR may be removed with or without cause by GTCR; provided, however, that at any time when GTCR controls less than 40% in voting power of our outstanding common stock, all directors, including those nominated by GTCR, may only be removed for cause, and only by the affirmative vote of holders of at least 66 2/3% in voting power of all the then-outstanding shares of capital stock of the company entitled to vote thereon, voting together as a single class. In addition, our certificate will also provide that, subject to the rights granted to one or more series of preferred stock then outstanding, any newly created directorship on our Board that results from an increase in the number of directors and any vacancies on our Board will be filled only by the affirmative vote of a majority of the remaining directors, even if less than a quorum, or by a sole remaining director (and not by the shareholders).
Supermajority Approval Requirements. Our certificate and bylaws will provide that our Board is expressly authorized to make, alter, amend, change, add to, rescind or repeal, in whole or in part, our bylaws without a shareholder vote in any matter not inconsistent with the laws of the State of Delaware and our certificate. For as long as GTCR controls, in the aggregate, at least 50% in voting power of our outstanding common stock, any amendment, alteration, rescission or repeal of our bylaws by our shareholders will require the affirmative vote of a majority in voting power of the outstanding shares of our stock entitled to vote on such amendment, alteration, change, addition, rescission or repeal. At any time when GTCR controls, in the aggregate, less than 50% in voting power of our outstanding common stock, any amendment, alteration, rescission or repeal of our bylaws by our shareholders will require the affirmative vote of the holders of at least 66 2/3% in voting power of all the then-outstanding shares of stock of the company entitled to vote thereon, voting together as a single class.
The DGCL provides generally that the affirmative vote of a majority of the outstanding shares entitled to vote thereon, voting together as a single class, is required to amend a corporation’s certificate of incorporation, unless the certificate requires a greater percentage.
Our certificate will provide that the following provisions in our certificate may be amended, altered, repealed or rescinded only by the affirmative vote of the holders of at least 66 2/3% (as opposed to a majority threshold) in voting power of all the then-outstanding shares of stock entitled to vote thereon, voting together as a single class:
| • | the provision requiring a 66 2/3% supermajority vote for shareholders to amend our bylaws; |
204
| • | the provisions providing for a classified board of directors (the election and term of our directors); |
| • | the provisions regarding resignation and removal of directors; |
| • | the provisions regarding entering into business combinations with interested shareholders; |
| • | the provisions regarding shareholder action by written consent; |
| • | the provisions regarding calling special meetings of shareholders; |
| • | the provisions regarding filling vacancies on our Board and newly created directorships; |
| • | the provision establishing the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation; |
| • | the provision establishing the federal district courts of the United States as the exclusive forum for litigation arising under the Securities Act; |
| • | the provisions eliminating monetary damages for breaches of fiduciary duty by a director; and |
| • | the amendment provision requiring that the above provisions be amended only with a 66 2/3% supermajority vote. |
The combination of the classification of our Board, the lack of cumulative voting and the supermajority voting requirements will make it more difficult for our existing shareholders to replace our Board as well as for another party to obtain control of us by replacing our Board. Because our Board has the power to retain and discharge our officers, these provisions could also make it more difficult for existing shareholders or another party to effect a change in management.
Authorized but Unissued Shares. Our authorized but unissued shares of common stock and preferred stock will be available for future issuance without shareholder approval, subject to stock exchange rules. These additional shares of capital stock may be utilized for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. One of the effects of the existence of authorized but unissued common stock or preferred stock may be to enable our Board to issue shares of capital stock to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of the company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our shareholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Business Combinations. Upon completion of this offering, we will not be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested shareholder” for a three-year period following the time that the person becomes an interested shareholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested shareholder. An “interested shareholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested shareholder status, 15% or more of the corporation’s voting stock.
Under Section 203, a business combination between a corporation and an interested shareholder is prohibited unless it satisfies one of the following conditions: (1) before the shareholder became an interested shareholder, the Board approved either the business combination or the transaction which resulted in the shareholder becoming an interested shareholder; (2) upon consummation of the transaction which resulted in the shareholder becoming an interested shareholder, the interested shareholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances; or (3) at or after the time the shareholder became an interested shareholder, the business combination was approved by the Board and authorized at an annual or special meeting
205
of the shareholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested shareholder.
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a shareholders’ amendment approved by at least a majority of the outstanding voting shares.
We will opt out of Section 203; however, our certificate will contain similar provisions providing that we may not engage in certain “business combinations” with any “interested shareholder” for a three-year period following the time that the shareholder became an interested shareholder, unless:
| • | prior to such time, our Board approved either the business combination or the transaction which resulted in the shareholder becoming an interested shareholder; |
| • | upon consummation of the transaction that resulted in the shareholder becoming an interested shareholder, the interested shareholder owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding certain shares; or |
| • | at or subsequent to that time, the business combination is approved by our Board and by the affirmative vote of holders of at least 66 2/3% of our outstanding voting stock that is not owned by the interested shareholder. |
Under certain circumstances, this provision will make it more difficult for a person who would be an “interested shareholder” to effect various business combinations with us for a three-year period. This provision may encourage companies interested in acquiring us to negotiate in advance with our Board because the shareholder approval requirement would be avoided if our Board approves either the business combination or the transaction which results in the shareholder becoming an interested shareholder. These provisions also may have the effect of preventing changes in our Board and may make it more difficult to accomplish transactions which shareholders may otherwise deem to be in their best interests.
Our certificate of incorporation will provide that GTCR, and any of its direct or indirect transferees and any group as to which such persons are a party, do not constitute “interested shareholders” for purposes of this provision.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their shareholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. Our certificate will include a provision that eliminates the personal liability of directors for monetary damages for any breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions will be to eliminate the rights of us and our shareholders, through shareholders’ derivative suits on our behalf, to recover monetary damages from a director for breach of fiduciary duty as a director, including breaches resulting from grossly negligent behavior. However, exculpation will not apply to any director if the director has acted in bad faith, knowingly or intentionally violated the law, authorized illegal dividends or redemptions or derived an improper benefit from his or her actions as a director.
Our bylaws will provide that we must indemnify and advance expenses to our directors and officers to the fullest extent authorized by the DGCL. We also will be expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers and certain employees for some liabilities. We believe that these indemnification and advancement provisions and insurance will be useful to attract and retain qualified directors and officers.
The limitation of liability, indemnification and advancement provisions that will be included in our certificate of incorporation and bylaws may discourage shareholders from bringing a lawsuit against directors for breaches of
206
their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our shareholders. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought.
Corporate Opportunity Doctrine
Delaware law permits corporations to adopt provisions renouncing any interest or expectancy in certain opportunities that are presented to the corporation or its officers, directors or shareholders. Our certificate will, to the maximum extent permitted from time to time by Delaware law, renounce any interest or expectancy that we have in, or right to be offered an opportunity to participate in, specified business opportunities that are from time to time presented to certain of our officers, directors or shareholders or their respective affiliates, other than those officers, directors, shareholders or affiliates who are our or our subsidiaries’ employees. Our certificate will provide that, to the fullest extent permitted by law, none of GTCR or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his or her director and officer capacities) or its, his or her affiliates will have any duty to refrain from (1) engaging in a corporate opportunity in the same or similar lines of business in which we or our affiliates now engage or propose to engage or (2) otherwise competing with us or our affiliates. In addition, to the fullest extent permitted by law, in the event that GTCR or any non-employee director acquires knowledge of a potential transaction or other business opportunity which may be a corporate opportunity for itself, himself or herself or its, his or her affiliates or for us or our affiliates, such person will have no duty to communicate or offer such transaction or business opportunity to us or any of our affiliates and they may take any such opportunity for themselves or offer it to another person or entity. Our certificate will not renounce our interest in any business opportunity that is expressly offered to a non-employee director solely in his or her capacity as a director or officer of Maravai LifeSciences Holdings, Inc. To the fullest extent permitted by law, no business opportunity will be deemed to be a potential corporate opportunity for us unless we would be permitted to undertake the opportunity under our certificate, we have sufficient financial resources to undertake the opportunity and the opportunity would be in line with our business.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, our shareholders will have appraisal rights in connection with a merger or consolidation of Maravai LifeSciences Holdings, Inc. Pursuant to the DGCL, shareholders who properly request and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares of capital stock as determined by the Delaware Court of Chancery.
Shareholders’ Derivative Actions
Under the DGCL, any of our shareholders may bring an action in our name to procure a judgment in our favor, also known as a derivative action, provided that the shareholder bringing the action is a holder of our shares of capital stock at the time of the transaction to which the action relates or such shareholder’s stock thereafter devolved by operation of law.
Transfer Agent and Registrar
The transfer agent and registrar for our Class A common stock will be American Stock Transfer & Trust Company, LLC. Its address is 6201 15th Avenue, Brooklyn, New York, 11219.
Listing
Our Class A common stock has been approved for listing on The Nasdaq Global Select Market under the trading symbol “MRVI.”
207
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our Class A common stock. Future sales of substantial amounts of our Class A common stock in the public market (including shares of our Class A common stock issuable upon redemption or exchange of LLC Units), or the perception that such sales may occur, could adversely affect the prevailing market price of our Class A common stock. No prediction can be made as to the effect, if any, future sales of shares, or the availability of shares for future sales, will have on the prevailing market price of our Class A common stock from time to time. The number of shares available for future sale in the public market is subject to legal and contractual restrictions, some of which are described below. The expiration of these restrictions will permit sales of substantial amounts of our Class A common stock in the public market, or could create the perception that these sales may occur, which could adversely affect the prevailing market price of our Class A common stock. These factors could also make it more difficult for us to raise funds through future offerings of Class A common stock or other equity or equity-linked securities.
Sale of Restricted Shares
Upon completion of this offering, we will have 81,379,818 shares of Class A common stock outstanding (87,747,455 shares if the underwriters exercise in full their option to purchase additional shares). Of these shares of Class A common stock, the 50,000,000 shares of Class A common stock being sold in this offering, plus any shares sold upon exercise of the underwriters’ option to purchase additional shares, will be freely tradable without restriction under the Securities Act of 1933, as amended (the “Securities Act”), except for any such shares which may be held or acquired by an “affiliate” of ours, as that term is defined in Rule 144 promulgated under the Securities Act (“Rule 144”), which shares will be subject to the volume limitations and other restrictions of Rule 144 described below. The remaining 31,379,818 shares of Class A common stock (or 207,838,510 shares of Class A common stock, including shares of Class A common stock issuable upon redemption or exchange of the LLC Units, as described below) will be “restricted securities,” as that phrase is defined in Rule 144, and may be resold only after registration under the Securities Act or pursuant to an exemption from such registration, including, among others, the exemptions provided by Rule 144 and 701 under the Securities Act, which rules are summarized below. These remaining shares of Class A common stock that will be outstanding upon completion of this offering will be available for sale in the public market after the expiration of market stand-off agreements with us and the lock-up agreements described in “Underwriters,” taking into account the provisions of Rules 144 and 701 under the Securities Act.
In addition, pursuant to the Exchange Agreement, MLSH 1 may from time to time after the consummation of this offering, exchange its LLC Units for shares of Class A common stock on a one-for-one basis, or, at our election, for cash, from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). MLSH 1 will also be required to deliver to us a number of shares of Class B common stock equivalent to the number of shares of Class A common stock being exchanged to effectuate an exchange. Any shares of Class B common stock so delivered will be cancelled. Upon consummation of this offering, MLSH 1 will hold 176,458,692 LLC Units, all of which will be exchangeable for shares of our Class A common stock or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). The shares of Class A common stock we issue upon such exchanges would be “restricted securities” as defined in Rule 144 unless we register such issuances. However, we intend to enter into a registration rights agreement with MLSH 1 that will require us to register these shares of Class A common stock, subject to certain conditions. See “—Registration Rights” and “Certain Relationships and Related Party Transactions—Registration Rights Agreement.”
Under the terms of the LLC Operating Agreement, except pursuant to a valid exchange under the terms of the Exchange Agreement, all of the LLC Units received by MLSH 1 in the Organizational Transactions will be subject to restrictions on disposition.
208
Rule 144
Persons who became the beneficial owner of shares of our Class A common stock prior to the completion of this offering may not sell their shares until the earlier of (1) the expiration of a six-month holding period, if we have been subject to the reporting requirements of the Exchange Act and have filed all required reports for at least 90 days prior to the date of the sale, or (2) a one-year holding period.
At the expiration of the six-month holding period, a person who was not one of our affiliates at any time during the three months preceding a sale would be entitled to sell an unlimited number of shares of our Class A common stock provided current public information about us is available, and a person who was one of our affiliates at any time during the three months preceding a sale would be entitled to sell within any three-month period only a number of shares of Class A common stock that does not exceed the greater of either of the following:
| • | 1% of the number of shares of our Class A common stock then outstanding, which will equal approximately 813,798 shares immediately after this offering, based on the number of shares of our Class A common stock outstanding after completion of this offering; or |
| • | the average weekly trading volume of our Class A common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
At the expiration of the one-year holding period, a person who was not one of our affiliates at any time during the three months preceding a sale would be entitled to sell an unlimited number of shares of our Class A common stock without restriction. A person who was one of our affiliates at any time during the three months preceding a sale would remain subject to the volume restrictions described above.
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us. The sale of these shares, or the perception that sales will be made, could adversely affect the price of our Class A common stock after this offering.
Rule 701
In general, under Rule 701, any of our employees, directors, officers, consultants or advisors who acquired shares of capital stock from us in connection with a compensatory stock or option plan or other compensatory written agreement before the effective date of this offering are, subject to applicable lock-up restrictions, eligible to resell such shares in reliance upon Rule 144 beginning 90 days after the date of this prospectus. If such person is not an affiliate and was not our affiliate at any time during the preceding three months, the sale may be made subject only to the manner-of-sale restrictions of Rule 144. If such a person is an affiliate, the sale may be made under Rule 144 without compliance with holding period requirements under Rule 144, but subject to the other Rule 144 restrictions described above.
Stock Plans
We intend to file one or more registration statements on Form S-8 under the Securities Act to register shares of our Class A common stock issued or reserved for issuance under the 2020 Plan. The first such registration statement is expected to be filed soon after the date of this prospectus and will automatically become effective upon filing with the SEC. Accordingly, shares of Class A common stock registered under such registration statement will be available for sale in the open market following the effective date, unless such shares are subject to vesting restrictions with us, Rule 144 restrictions applicable to our affiliates or the lock-up restrictions described below.
Lock-Up Agreements
We, each of our officers and directors and other shareholders and optionholders owning substantially all of our Class A common stock and options or other securities to acquire Class A common stock have agreed that,
209
without the prior written consent of the representatives on behalf of the underwriters, we and they will not, subject to limited exceptions, directly or indirectly sell or dispose of any of the shares of Class A common stock or securities convertible into or exchangeable for, or that represent the right to receive, shares of common stock, including LLC Units, during the period from the date of the first public filing of the registration statement on Form S-1 filed in connection with this offering continuing through the date that is 180 days after the date of this prospectus. The lock-up restrictions and specified exceptions are described in more detail under “Underwriters.” The representatives may, in their discretion, release all or any portion of the securities subject to these lock-up agreements. See “Underwriters.”
Prior to the consummation of the offering, certain of our employees, including our executive officers, and/or directors may enter into written trading plans that are intended to comply with Rule 10b5-1 under the Exchange Act. Sales under these trading plans would not be permitted until the expiration of the lock-up agreements relating to the offering described above.
Following the lock-up periods set forth in the agreements described above, and assuming that the representatives of the underwriters do not release any parties from these agreements, all of the shares of our Class A common stock that are restricted securities or are held by our affiliates as of the date of this prospectus will be eligible for sale in the public market in compliance with Rule 144 under the Securities Act.
Registration Rights Agreement
We intend to enter into a Registration Rights Agreement with MLSH 1 and MLSH 2 in connection with this offering. The Registration Rights Agreement will provide MLSH 1 and MLSH 2 certain registration rights whereby, following our initial public offering and the expiration of any related lock-up period, MLSH 1 and MLSH 2 can require us to register under the Securities Act shares of Class A common stock (including shares issuable to MLSH 1 upon exchange of its LLC Units). The Registration Rights Agreement will also provide for piggyback registration rights for MLSH 1 and MLSH 2. See “Certain Relationships and Related Party Transactions—Registration Rights Agreement.”
210
MATERIAL U.S. FEDERAL INCOME TAX
CONSEQUENCES TO NON-U.S. HOLDERS
The following discussion is a summary of the material U.S. federal income tax consequences to Non-U.S. Holders (as defined below) of the ownership and disposition of our Class A common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax consequences. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or non-U.S. tax laws are not discussed. This discussion is based on the Code, Treasury regulations promulgated thereunder (the ”Treasury Regulations”), judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service (the “IRS”), in each case as in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a Non-U.S. Holder of our common stock. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position to those discussed below regarding the tax consequences of the purchase, ownership and disposition of our Class A common stock.
This discussion is limited to Non-U.S. Holders that hold our Class A common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Non-U.S. Holder’s particular circumstances, including the impact of the Medicare tax on net investment income or the alternative minimum tax, or the consequences to persons subject to special tax accounting rules as a result of any item of gross income with respect to our Class A common stock being taken into account in an applicable financial statement. In addition, it does not address consequences relevant to Non-U.S. Holders subject to special rules, including, without limitation:
| • | U.S. expatriates and former citizens or long-term residents of the U.S.; |
| • | persons holding our common stock as part of a straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| • | banks, insurance companies and other financial institutions; |
| • | real estate investment trusts or regulated investment companies; |
| • | brokers, dealers or certain electing traders in securities that mark their securities positions to market for tax purposes; |
| • | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | tax-exempt organizations or governmental organizations; |
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| • | “qualified foreign pension funds” (within the meaning of Section 897(I)(2) of the Code and entities, all of the interests of which are held by qualified foreign pension funds); and |
| • | tax-qualified retirement plans. |
If any partnership or arrangement classified as a partnership for U.S. federal income tax purposes holds our Class A common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our Class A common stock and partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
211
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE OWNERSHIP AND DISPOSITION OF OUR CLASS A COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Definition of a Non-U.S. Holder
For purposes of this discussion, a “Non-U.S. Holder” is any beneficial owner of our Class A common stock that is neither a “United States person” nor an entity treated as a partnership for U.S. federal income tax purposes. A United States person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| • | an individual who is a citizen or resident of the U.S.; |
| • | a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized under the laws of the U.S. any state thereof, or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
Distributions
As described in the section entitled “Dividend Policy,” we do not anticipate declaring or paying dividends to holders of our Class A common stock in the foreseeable future. However, if we do make distributions of cash or property on our Class A common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will first constitute non-taxable returns of capital and be applied against and reduce a Non-U.S. Holder’s adjusted tax basis in its Class A common stock, but not below zero. Any excesses will be treated as capital gains and will be treated as described below under “Sale or Other Taxable Disposition.”
Subject to the discussion below on effectively connected income, backup withholding, and the Foreign Account Tax Compliance Act, dividends paid to a Non-U.S. Holder of our Class A common stock will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty, provided that the Non-U.S. Holder will be required to furnish to the applicable withholding agent prior to the payment of dividends a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate in order to avoid withholding with respect to such tax). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
If dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the U.S. to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the Non-U.S. Holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S.
212
Any such effectively connected dividends will be subject to U.S. federal income tax on a net-income basis at the regular graduated rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits (as adjusted for certain items), which will include such effectively connected dividends. Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Sale or Other Taxable Disposition
Subject to the discussion below on backup withholding and the Foreign Account Tax Compliance Act, a Non-U.S. Holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale or other taxable disposition of our Class A common stock unless:
| • | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the U.S. to which such gain is attributable); |
| • | the Non-U.S. Holder is a nonresident alien individual present in the U.S. for 183 days or more during the taxable year of the disposition and certain other requirements are met; or |
| • | our common stock constitutes a U.S. real property interest (a “USRPI”), by reason of our status as a U.S. real property holding corporation (a “USRPHC”) for U.S. federal income tax purposes. |
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the regular graduated rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits (as adjusted for certain items), which will include such effectively connected gain.
A Non-U.S. Holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on any gain derived from the disposition, which may generally be offset by U.S. source capital losses of the Non-U.S. Holder (even though the individual is not considered a resident of the U.S.), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we currently are not, and do not anticipate becoming, a USRPHC. Because the determination of whether we are a USRPHC depends, however, on the fair market value of our USRPIs relative to the fair market value of our non-U.S. real property interests and our other business assets, there can be no assurance we currently are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition by a Non-U.S. Holder of our common stock will not be subject to U.S. federal income tax if our Class A common stock is “regularly traded on an established securities market,” as defined by applicable Treasury Regulations, during the calendar year in which the disposition occurs, and such Non-U.S. Holder has owned, actually and constructively, five percent or less of our Class A common stock throughout the shorter of (1) the five-year period ending on the date of the sale or other taxable disposition and (2) the Non-U.S. Holder’s holding period. If we were to become a USRPHC and our Class A common stock were not considered to be “regularly traded on an established securities market” during the calendar year in which the relevant disposition by a Non-U.S. Holder occurred, such Non-U.S. Holder (regardless of the percentage of stock owned) would be subject to U.S. federal income tax on a sale or other taxable disposition of our Class A common stock and a 15% withholding tax would apply to the gross proceeds from such disposition.
Non-U.S. Holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
213
Information Reporting and Backup Withholding
Payments of dividends on our Class A common stock generally will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know the Non-U.S. Holder is a United States person and the Non-U.S. Holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any dividends on our Class A common stock paid to the Non-U.S. Holder, regardless of whether any tax was actually withheld. In addition, proceeds of the sale or other taxable disposition of our common stock within the U.S. or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such Non-U.S. Holder is a United States person, or the Non-U.S. Holder otherwise establishes an exemption. If a Non-U.S. Holder does not provide the certification described above or the applicable withholding agent has actual knowledge or reason to know that such Non-U.S. Holder is a United States person, payments of dividends or of proceeds of the sale or other taxable disposition of our common stock may be subject to backup withholding at a rate currently equal to 24% of the gross proceeds of such dividend, sale, or taxable disposition. Proceeds of a disposition of our Class A common stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides or is established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts
Sections 1471 through 1474 of the Code and the Treasury regulations and administrative guidance promulgated thereunder (commonly referred to as the “Foreign Account Tax Compliance Act” or “FATCA”) generally impose withholding at a rate of 30% in certain circumstances on dividends in respect of securities which are held by or through certain foreign financial institutions (including investment funds), unless any such institution (i) enters into, and complies with, an agreement with the IRS to report, on an annual basis, information with respect to interests in, and accounts maintained by, the institution that are owned by certain U.S. persons and by certain non-U.S. entities that are wholly or partially owned by U.S. persons and to withhold on certain payments, or (ii) if required under an intergovernmental agreement between the United States and an applicable foreign country, reports such information to its local tax authority, which will exchange such information with the U.S. authorities. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Accordingly, the entity through which shares of our Class A common stock are held will affect the determination of whether such withholding is required. Similarly, dividends in respect of our Class A common stock held by an investor that is a non-financial non-U.S. entity that does not qualify under certain exceptions will generally be subject to withholding at a rate of 30%, unless such entity either (i) certifies to the applicable withholding agent that such entity does not have any “substantial United States owners” or (ii) provides certain information regarding the entity’s “substantial United States owners,” which will in turn be provided to the U.S. Department of Treasury. Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our Class A common stock.
214
Under the terms and subject to the conditions in an underwriting agreement dated the date of this prospectus, the underwriters named below, for whom Morgan Stanley & Co. LLC, Jefferies LLC and Goldman Sachs & Co. LLC are acting as representatives, have severally agreed to purchase, and we have agreed to sell to them, severally, the number of shares of Class A common stock indicated below:
| Name |
Number of Shares |
|||
| Morgan Stanley & Co. LLC |
||||
| Jefferies LLC |
||||
| Goldman Sachs & Co. LLC |
||||
| BofA Securities, Inc. |
||||
| Credit Suisse Securities (USA) LLC |
||||
| UBS Securities LLC |
||||
| Robert W. Baird & Co. Incorporated |
||||
| William Blair & Company, L.L.C. |
||||
| Stifel, Nicolaus & Company, Incorporated |
||||
| KeyBanc Capital Markets Inc. |
||||
| Academy Securities, Inc. |
||||
| Loop Capital Markets LLC |
||||
| Penserra Securities LLC |
||||
| Tigress Financial Partners LLC |
||||
|
|
|
|||
| Total: |
50,000,000 | |||
|
|
|
|||
The underwriters and the representatives are collectively referred to as the “underwriters” and the “representatives,” respectively. The underwriters are offering the shares of Class A common stock subject to their acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the shares of Class A common stock offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the shares of Class A common stock offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares of Class A common stock covered by the underwriters’ option to purchase additional shares described below.
The underwriters initially propose to offer part of the shares of Class A common stock directly to the public at the offering price listed on the cover page of this prospectus and part to certain dealers at a price that represents a concession not in excess of $ per share under the public offering price. After the initial offering of the shares of Class A common stock, the offering price and other selling terms may from time to time be varied by the representatives.
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to 7,500,000 additional shares of Class A common stock at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to purchase about the same percentage of the additional shares of Class A common stock as the number listed next to the underwriter’s name in the preceding table bears to the total number of shares of Class A common stock listed next to the names of all underwriters in the preceding table.
215
The following table shows the per share and total public offering price, underwriting discounts and commissions, and proceeds before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Total | ||||||||||||
| Per Share |
No Exercise |
Full Exercise |
||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions to be paid by us |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
Perella Weinberg Partners LP (“Perella Weinberg”), a Financial Industry Regulatory Association, Inc. (“FINRA”) member, is acting as our financial advisor in connection with the offering. We expect to pay Perella Weinberg, upon the successful completion of this offering, a fee of approximately $9.4 million for its services. The services provided to us by Perella Weinberg include, among other things, an independent financial valuation analysis; assisting in drafting our positioning and investment thesis; assisting us in our interactions with the underwriters; and assisting us in crafting an appropriate aftermarket trading and investor relations strategy. Apart from Perella Weinberg’s role as financial advisor in connection with this offering, we have had no other relationships with Perella Weinberg. Perella Weinberg will not sell or offer to sell any securities in this offering and will not identify, solicit or engage directly with potential investors in this offering. In addition, Perella Weinberg will not purchase any of the offered shares of Class A common stock.
The estimated offering expenses payable by us, exclusive of the underwriting discounts and commissions, are approximately $20.4 million, including the $9.4 million fee payable to Perella Weinberg discussed above. We have agreed to reimburse the underwriters for expense relating to clearance of this offering with FINRA up to $50,000.
The underwriters have informed us that they do not intend sales to discretionary accounts to exceed 5% of the total number of shares of Class A common stock offered by them.
Our Class A common stock has been approved for listing on The Nasdaq Global Select Market under the trading symbol “MRVI.”
We and all directors and officers and the holders of substantially all of our outstanding stock and stock options have agreed that, without the prior written consent of the representatives on behalf of the underwriters, we and they will not, and will not publicly disclose an intention to or cause any affiliate to, during the period ending 180 days after the date of this prospectus (the “restricted period”):
| • | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, including LLC Units (“Lock-Up Securities”); |
| • | file or confidentially submit any registration statement with the SEC relating to the offering of any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; or |
| • | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any Lock-Up Securities, |
whether any such transaction described above is to be settled by delivery of common stock or such other securities, in cash or otherwise. In addition, we and each such person agrees that, without the prior written consent of the representatives on behalf of the underwriters, we or such other person will not, during the restricted period, make any demand for, or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock.
216
The restrictions described in the immediately preceding paragraph to do not apply to our directors and officers or holders of our outstanding Class A common stock or other securities in certain circumstances, including:
| • | transactions relating to shares of Class A common stock acquired in open market transactions after the completion of the offering of the shares, provided that no filing under Section 16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is required or voluntarily made in connection with subsequent sales of the Class A common stock or other securities acquired in such open market transactions; |
| • | facilitating the establishment of a trading plan on behalf of a shareholder, officer or director of Maravai LifeSciences Holdings, Inc. pursuant to Rule 10b5-1 under the Exchange Act for the transfer of shares of Class A common stock, provided that such plan does not provide for the transfer of Class A common stock during the restricted period and provided further that to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made by Maravai LifeSciences Holdings, Inc. regarding the establishment of such plan, such announcement or filing shall include a statement to the effect that no transfer of Class A common stock may be made under such plan during the restricted period; |
| • | transfers of Lock-Up Securities as a bona fide gift or gifts, to an immediate family member, to certain trusts or to a corporation, partnership, limited liability company, trust or other entity of which the holder and the immediate family of the holder are the legal and beneficial owner of all of the outstanding equity securities or similar interests, provided that each donee or transferee signs a lock-up agreement and provided further that certain filings under Section 16(a) of the Exchange Act or any other public filing or disclosure reporting a reduction in beneficial ownership of common stock shall not be required or voluntarily made during the restricted period; |
| • | distributions of Lock-Up Securities to partners, members or stockholders, wholly-owned subsidiaries or affiliates of the holder, or if such transferee is not a natural person, to any direct or indirect partners, members or shareholders of such transferee until the Lock-Up Securities come to be held by a natural person, provided that certain transferees or distributees sign a lock-up agreement and provided further that any filing under Section 16(a) of the Exchange Act shall clearly indicate in the footnotes thereto the nature and conditions of such transfer, that such transfer is not for value and that the Lock-Up Securities subject to such transfer are subject to a lock-up agreement; |
| • | transfers of Lock-Up Securities pursuant to a bona fide third party tender offer, merger, consolidation or other similar transaction or transactions made to all or substantially all holders of our common stock and approved by our board of directors, provided that in the event that such tender offer, merger, consolidation or other such transaction or transactions shall not be completed, the holder’s Lock-Up Securities shall remain subject to the provisions of the lock-up agreement during the restricted period; |
| • | transfers of Lock-Up Securities as a result of the operation of law, pursuant to an order of a court or regulatory agency or by will, other testamentary document or intestate succession, provided that certain filings under Section 16(a) of the Exchange Act or any other public filing or disclosure reporting a reduction in beneficial ownership of common stock shall not be required or voluntarily made during the restricted period; |
| • | repurchases of Lock-Up Securities pursuant to equity award agreements or other contractual arrangements providing for the right of such repurchase in connection with the termination of the holders employment or service with us, provided that no filing by the holder under the Exchange Act, or other public announcement, shall be voluntarily made in connection with any such transfer, and if the holder is required to file a report under the Exchange Act related thereto during the restricted period, such report shall disclose that such transfer was a result of our repurchase of the Lock-Up Securities pursuant to equity award agreements or other contractual arrangements in connection with the termination of the holders’s employment or service with us; |
| • | receipt of Lock-Up Securities upon the exercise of an option to purchase Lock-Up Securities in connection with the termination or expiration of such option pursuant to its terms, provided that such |
217
| option was granted pursuant to a stock option plan or other equity incentive plan described in this prospectus, provided further that any Lock-Up Securities received upon such exercise shall be subject to the lock-up agreement and provided further that no filing under Section 16(a) of the Exchange Act or other public filing, report or announcement reporting a reduction in the aggregate beneficial ownership of the Lock-Up Securities shall be required or shall be voluntarily made within 90 days after the date of this prospectus, and after such 90th day, any required report under Section 16(a) of the Exchange Act shall clearly indicate in the footnotes that the Lock-Up Securities issuable upon such exercise are subject to a lock-up agreement and that the filing relates to such circumstances and provided further that no other public filing or announcement shall be required or shall be made voluntarily in connection with such exercise; |
| • | transfers of Lock-Up Securities to us pursuant to the exercise, on a “cashless” or “net exercise” basis, of any option to purchase Lock-Up Securities granted pursuant to stock option or equity incentive plans described in this prospectus, or for the purpose of satisfying any withholding taxes due as a result of the exercise of any option to purchase Lock-Up Securities or the vesting of any equity awards granted pursuant to stock option or equity incentive plans described in this prospectus, provided that no filing under the Exchange Act, or other public announcement, shall be voluntarily made in connection with any such transfer, and if the holder is required to file a report under the Exchange Act related thereto during the restricted period, such report shall disclose that such transfer was a result of such circumstances; and |
| • | in any exchange of LLC Units and a corresponding number of shares of our Class B common stock into or for shares of Class A common stock (or securities convertible into or exercisable or exchangeable for Class A common stock) in a manner consistent with the Exchange Agreement; provided that to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made regarding the exchange, such announcement or filing shall include a statement to the effect that such exchange occurred pursuant to the Exchange Agreement and provided further that no transfer of the shares of Class A common stock or other securities received upon exchange may be made during the restricted period. |
The restrictions also do not apply to us in certain circumstances, including:
| • | the sale of shares to the underwriters in this offering; |
| • | the issuance by us of shares of common stock upon the exercise of an option or a warrant or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing; |
| • | the sale or issuance of or entry into an agreement providing for the sale or issuance of common stock or securities convertible into, exercisable for or which are otherwise exchangeable for or represent the right to receive common stock in connection with the acquisition of securities, businesses, technology, property or other assets; joint ventures; commercial relationships or other strategic transactions, provided that the aggregate number of shares of common stock securities convertible into, exercisable for or which are otherwise exchangeable for or represent the right to receive common stock that we may sell or issue or agree to sell or issue pursuant to such circumstances shall not exceed % of the total number of shares of common stock outstanding immediately following the completion of the transactions contemplated by this prospectus to be completed as of that date, and provided further that all recipients of any such securities shall enter into a lock-up agreement for the remainder of the restricted period; |
| • | facilitating the establishment of a trading plan on behalf of a shareholder, officer or director of Maravai LifeSciences Holdings, Inc. pursuant to Rule 10b5-1 under the Exchange Act for the transfer of shares of Class A common stock, provided that such plan does not provide for the transfer of Class A common stock during the restricted period and provided further that to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made by us regarding the |
218
| establishment of such plan, such announcement or filing shall include a statement to the effect that no transfer of Class A common stock may be made under such plan during the restricted period, and |
| • | the filing by us of a registration statement on Form S-8 relating to securities granted or to be granted pursuant to any compensation benefit plan described in this prospectus. |
The representatives, in their discretion, may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time.
In order to facilitate the offering of the Class A common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the Class A common stock. Specifically, the underwriters may sell more shares than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for purchase by the underwriters under the option to purchase additional shares described above. The underwriters can close out a covered short sale by exercising the option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the underwriters will consider, among other things, the open market price of shares compared to the price available under the option to purchase additional shares. The underwriters may also sell shares in excess of the option to purchase additional shares, creating a naked short position. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the Class A common stock in the open market after pricing that could adversely affect investors who purchase in this offering. As an additional means of facilitating this offering, the underwriters may bid for, and purchase, shares of Class A common stock in the open market to stabilize the price of the Class A common stock. These activities may raise or maintain the market price of the Class A common stock above independent market levels or prevent or retard a decline in the market price of the Class A common stock. The underwriters are not required to engage in these activities and may end any of these activities at any time.
We and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
A prospectus in electronic format may be made available on websites maintained by one or more underwriters, or selling group members, if any, participating in this offering. The representatives may agree to allocate a number of shares of Class A common stock to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters that may make Internet distributions on the same basis as other allocations.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for us, for which they received or will receive customary fees and expenses.
In addition, in the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities and instruments. Such investment and securities activities may involve our securities and instruments. The underwriters and their respective affiliates may also make investment recommendations or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long or short positions in such securities and instruments.
219
Pricing of the Offering
Prior to this offering, there has been no public market for our Class A common stock. The initial public offering price was determined by negotiations between us and the representatives. Among the factors considered in determining the initial public offering price were our future prospects and those of our industry in general, our sales, earnings and certain other financial and operating information in recent periods, and the price-earnings ratios, price-sales ratios, market prices of securities, and certain financial and operating information of companies engaged in activities similar to ours.
Directed Share Program
At our request, the underwriters have reserved up to 1,725,000 shares of our Class A common stock, or 3% of the shares of our Class A common stock to be offered by this prospectus for sale, at the initial public offering price, for sale to certain individuals through a directed share program, including our directors, certain employees and certain other individuals identified by management. Shares purchased through the directed share program will not be subject to a lock-up restriction, except in the case of shares purchased by any of our directors or officers and certain of our employees and existing equityholders. The number of shares of Class A common stock available for sale to the general public will be reduced to the extent these individuals or entities purchase such reserved shares. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same basis as the other shares offered by this prospectus. The underwriters will receive the same discount from such reserved shares as they will from other shares of our Class A common stock sold to the public in this offering. We have agreed to indemnify the underwriters against certain liabilities and expenses, including liabilities under the Securities Act, in connection with sales of the reserved shares.
Selling Restrictions
European Economic Area and United Kingdom
In relation to each Member State of the European Economic Area and the United Kingdom (each a “Relevant State”), no shares of our Class A common stock have been offered or will be offered pursuant to this offering to the public in that Relevant State prior to the publication of a prospectus in relation to shares of our Class A common stock which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that offers of shares of our Class A common stock may be made to the public in that Relevant State at any time under the following exemptions under the Prospectus Regulation:
| • | to any legal entity which is a qualified investor as defined in the Prospectus Regulation; |
| • | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior consent of the representatives for any such offer; or |
| • | In any other circumstances falling within Article 1(4) of the Prospectus Regulation; |
provided that no such offer of shares of our Class A common stock shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to any shares of our Class A common stock in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares of our Class A common stock to be offered so as to enable an investor to decide to purchase or subscribe for any shares of our Class A common stock, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
This selling restriction is in addition to any other selling restrictions set out below.
220
United Kingdom
Each underwriter has represented and agreed that:
| (a) | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (“FSMA”)) received by it in connection with the issue or sale of the shares of Class A common stock in circumstances in which Section 21(1) of the FSMA does not apply to the Company; and |
| (b) | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of Class A common stock in, from or otherwise involving the United Kingdom. |
In the United Kingdom, this prospectus is only addressed to and directed at qualified investors who are (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the Order); or (ii) high net worth entities and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). Any investment or investment activity to which this prospectus relates is available only to relevant persons and will only be engaged with relevant persons. Any person who is not a relevant person should not act or rely on this prospectus or any of its contents.
Canada
The Class A common stock may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the Class A common stock must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Hong Kong
The Class A common stock may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), (ii) to “professional investors” as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the Class A common stock may be issued or may be in the possession of any
221
person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares of Class A common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
Japan
The shares of Class A common stock have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended) (“FIEA”). The shares of Class A common stock may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the Class A common stock may not be circulated or distributed, nor may the Class A common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”)) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the shares of Class A common stock are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for 6 months after that corporation has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”).
Where the shares of Class A common stock are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for 6 months after that trust has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32.
222
Solely for the purposes of our obligations pursuant to Section 309B of the SFA, we have determined, and hereby notify all relevant persons (as defined in the Securities and Futures (Capital Markets Products) Regulations 2018 (“CMP Regulations”)) that the shares of Class common stock are “prescribed capital markets products” (as defined in the CMP Regulations) and Excluded Investment Products (as defined in MAS Notice SFA 04-N12: Notice on the Sale of Investment Products and MAS Notice FAA-N16: Notice on Recommendations on Investment Products).
Switzerland
This prospectus is not intended to constitute an offer or solicitation to purchase or invest in the Class A common stock. The Class A common stock may not be publicly offered, directly or indirectly, in Switzerland within the meaning of the Swiss Financial Services Act (“FinSA”) and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading venue (exchange or multilateral trading facility) in Switzerland. This document does not constitute a prospectus within the meaning of, and has been prepared without regard to, the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading venue (exchange or multilateral trading facility) in Switzerland. Neither this document nor any other offering or marketing material relating to the Class A common stock constitutes a prospectus pursuant to the FinSA, and neither this document nor any other offering or marketing material relating to the Class A common stock or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, or the Class A common stock have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of Class A common stock will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of Class A common stock has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of Class A common stock.
United Arab Emirates
The Class A common stock has not been, and are not being, publicly offered, sold, promoted or advertised in the United Arab Emirates (including the Dubai International Financial Centre) other than in compliance with the laws of the United Arab Emirates (and the Dubai International Financial Centre) governing the issue, offering and sale of securities. Further, this prospectus does not constitute a public offer of securities in the United Arab Emirates (including the Dubai International Financial Centre) and is not intended to be a public offer. This prospectus has not been approved by or filed with the Central Bank of the United Arab Emirates, the Securities and Commodities Authority or the Dubai Financial Services Authority.
223
The validity of the issuance of our Class A common stock offered in this prospectus will be passed upon for us by Kirkland & Ellis LLP, Chicago, Illinois. Certain legal matters will be passed upon for the underwriters by Davis Polk & Wardwell LLP, Menlo Park, California.
The financial statements of Maravai LifeSciences Holdings, Inc. at September 1, 2020 appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The consolidated financial statements of Maravai Topco Holdings, LLC and subsidiaries as of December 31, 2018 and 2019, and for each of the two years in the period ended December 31, 2019, appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
224
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act to register our Class A common stock being offered in this prospectus. This prospectus, which forms part of the registration statement, does not contain all of the information included in the registration statement and the attached exhibits. You will find additional information about us and our Class A common stock in the registration statement. References in this prospectus to any of our contracts, agreements or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contracts, agreements or documents.
The SEC maintains a website that contains reports, proxy statements and other information about companies like us, who file electronically with the SEC. The address of that website is http://www.sec.gov. This reference to the SEC’s website is an inactive textual reference only and is not a hyperlink.
Upon the effectiveness of the registration statement, we will be subject to the reporting, proxy and information requirements of the Exchange Act, and will be required to file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information will be available on the website of the SEC referred to above, as well as on our website, https://www.maravai.com. This reference to our website is an inactive textual reference only and is not a hyperlink. The contents of, or other information accessible through, our website are not part of this prospectus, and you should not consider the contents of our website in making an investment decision with respect to our Class A common stock. We will furnish our shareholders with annual reports containing audited financial statements and quarterly reports containing unaudited interim financial statements for each of the first three quarters of each year.
225
| Page | ||||
| MARAVAI LIFESCIENCES HOLDINGS, INC. |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES |
||||
| Audited Financial Statements |
||||
| F-5 | ||||
| Consolidated Balance Sheets as of December 31, 2018 and 2019 |
F-6 | |||
| Consolidated Statements of Operations for the years ended December 31, 2018 and 2019 |
F-7 | |||
| Consolidated Statements of Comprehensive Loss for the years ended December 31, 2018 and 2019 |
F-8 | |||
| Consolidated Statements of Member’s Equity for the years ended December 31, 2018 and 2019 |
F-9 | |||
| Consolidated Statements of Cash Flows for the years ended December 31, 2018 and 2019 |
F-10 | |||
| Notes to Consolidated Financial Statements for the years ended December 31, 2018 and 2019 |
F-11 | |||
| Unaudited Interim Condensed Consolidated Financial Statements | ||||
| Condensed Consolidated Balance Sheets as of December 31, 2019 and September 30, 2020 |
F-45 | |||
| F-46 | ||||
| F-47 | ||||
| F-48 | ||||
| F-49 | ||||
| F-50 | ||||
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholder and the Board of Directors of Maravai LifeSciences Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheet of Maravai LifeSciences Holdings, Inc. (the Company) as of September 1, 2020 and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at September 1, 2020 in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2020.
Redwood City, California
September 8, 2020,
except as to the second paragraph of Note 3, as to which the date is
November 12, 2020
F-2
MARAVAI LIFESCIENCES HOLDINGS, INC.
As of September 1, 2020
| ASSETS |
||||
| Cash |
$ | 10 | ||
|
|
|
|||
| Total assets |
$ | 10 | ||
|
|
|
|||
| Commitments and contingencies |
||||
| STOCKHOLDER’S EQUITY: |
||||
| Common stock, $0.01 par value per share, 1,000 shares authorized, issued and outstanding |
$ | 10 | ||
|
|
|
|||
| Total Stockholder’s equity |
$ | 10 | ||
|
|
|
|||
The accompanying notes are an integral part of the financial statement.
F-3
MARAVAI LIFESCIENCES HOLDINGS, INC.
1. Organization
Maravai LifeSciences Holdings, Inc. (the “Company”) was formed as a Delaware corporation on August 25, 2020. The Company was formed for the purpose of completing a public offering and related transactions in order to carry on the business of Maravai Topco Holdings, LLC and its subsidiaries (“Topco LLC”). As the manager of Topco LLC, the Company is expected to operate and control all of the business and affairs of Topco LLC, and through Topco LLC, continue to conduct the business now conducted by these subsidiaries.
2. Summary of Significant Accounting Policies
Basis of Presentation and Accounting
The financial statement has been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). Separate statements of operations, comprehensive income, changes in stockholder’s equity, and cash flows have not been presented because there have been no activities in this entity as of September 1, 2020.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the balance sheet. Actual results could differ from those estimates.
3. Common Stock
On August 25, 2020, the Company was authorized to issue 1,000 shares of common stock, par value $0.01 per share, all of which have been issued or are outstanding. As of the balance sheet date, we issued, for $10.00, and had outstanding 1,000 shares all of which were owned by Maravai Life Sciences Holdings, LLC.
On October 20, 2020, the Company amended and restated its certificate of incorporation to create and authorize 50,000,000 shares of Class A common stock with a par value of $0.01 per share. On November 12, 2020 the Company amended and restated its certificate of incorporation to increase its authorized shares of Class A common stock from 50,000,000 to 500,000,000 shares.
4. Subsequent Events
The Company has evaluated subsequent events through September 8, 2020 (except for the creation and authorization of Class A common stock, as discussed in the second paragraph of Note 3, as to which the date is November 12, 2020), which is the date on which the financial statements were issued.
F-4
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Member and the Board of Directors of Maravai Topco Holdings, LLC and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Maravai Topco Holdings, LLC and Subsidiaries (the Company) as of December 31, 2018 and 2019, the related consolidated statements of operations, comprehensive loss, changes in member’s equity and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2018 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2016.
Redwood City, California
September 8, 2020,
except as to the fifth paragraph of Note 1, as to which the date is
November 11, 2020
F-5
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
(in thousands, except unit amounts)
| December 31, | ||||||||
| 2018 | 2019 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash |
$ | 21,866 | $ | 24,700 | ||||
| Accounts receivable, net |
15,991 | 18,030 | ||||||
| Inventory |
14,308 | 14,202 | ||||||
| Prepaid expenses and other current assets |
1,637 | 3,620 | ||||||
|
|
|
|
|
|||||
| Total current assets |
53,802 | 60,552 | ||||||
| Property and equipment, net |
42,578 | 94,311 | ||||||
| Goodwill |
224,275 | 224,275 | ||||||
| Intangible assets, net |
218,127 | 197,853 | ||||||
| Other assets |
894 | 805 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 539,676 | $ | 577,796 | ||||
|
|
|
|
|
|||||
| Liabilities and member’s equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 3,604 | $ | 7,478 | ||||
| Accrued expenses |
11,147 | 18,515 | ||||||
| Deferred revenue |
1,087 | 841 | ||||||
| Other current liabilities |
207 | 228 | ||||||
| Current portion of contingent consideration |
2,000 | — | ||||||
| Construction payable |
15,374 | — | ||||||
| Current portion of long-term debt |
2,500 | 2,500 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
35,919 | 29,562 | ||||||
| Long-term contingent consideration |
1,678 | — | ||||||
| Long-term debt, less current portion |
335,550 | 334,783 | ||||||
| Deferred tax liabilities |
15,856 | 14,697 | ||||||
| Lease facility financing obligation, less current portion |
1,839 | 52,919 | ||||||
| Other long-term liabilities |
818 | 1,208 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
391,660 | 433,169 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 6) |
||||||||
| Member’s equity |
||||||||
| Contributed capital, 253,916,941 units authorized, issued and outstanding |
182,809 | 183,910 | ||||||
| Accumulated deficit |
(38,237 | ) | (42,381 | ) | ||||
| Accumulated other comprehensive loss |
(167 | ) | (133 | ) | ||||
|
|
|
|
|
|||||
| Total member’s equity attributable to Maravai Topco Holdings, LLC member |
144,405 | 141,396 | ||||||
| Noncontrolling interests |
3,611 | 3,231 | ||||||
|
|
|
|
|
|||||
| Total member’s equity |
148,016 | 144,627 | ||||||
|
|
|
|
|
|||||
| Total liabilities and member’s equity |
$ | 539,676 | $ | 577,796 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the consolidated financial statements
F-6
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands except unit and per unit amounts)
| Year Ended December 31, | ||||||||
| 2018 | 2019 | |||||||
| Revenue |
$ | 123,833 | $ | 143,140 | ||||
| Operating expenses |
||||||||
| Cost of revenue |
60,765 | 66,849 | ||||||
| Research and development |
4,499 | 3,627 | ||||||
| Selling, general and administrative |
41,194 | 48,354 | ||||||
| Change in estimated fair value of contingent consideration |
939 | 322 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
107,397 | 119,152 | ||||||
|
|
|
|
|
|||||
| Income from operations |
16,436 | 23,988 | ||||||
| Other income (expense) |
||||||||
| Interest expense |
(27,399 | ) | (29,959 | ) | ||||
| Loss on extinguishment of debt |
(5,622 | ) | — | |||||
| Other income |
87 | 118 | ||||||
|
|
|
|
|
|||||
| Loss before income taxes |
(16,498 | ) | (5,853 | ) | ||||
| Income tax expense (benefit) |
417 | (652 | ) | |||||
|
|
|
|
|
|||||
| Net loss |
(16,915 | ) | (5,201 | ) | ||||
| Net loss attributable to noncontrolling interests |
(12,443 | ) | (731 | ) | ||||
|
|
|
|
|
|||||
| Net loss attributable to the Maravai Topco Holdings, LLC member |
$ | (4,472 | ) | $ | (4,470 | ) | ||
|
|
|
|
|
|||||
| Net loss per common unit attributable to Maravai Topco Holdings, LLC member—basic and diluted |
$ | (0.07 | ) | $ | (0.03 | ) | ||
|
|
|
|
|
|||||
| Weighted average common units outstanding |
253,916,941 | 253,916,941 | ||||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the consolidated financial statements
F-7
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
Years Ended December 31, 2018 and 2019
(in thousands)
| Year Ended December 31, | ||||||||
| 2018 | 2019 | |||||||
| Net loss |
$ | (16,915 | ) | $ | (5,201 | ) | ||
| Other comprehensive loss |
||||||||
| Foreign currency translation adjustments, net of tax |
(69 | ) | 34 | |||||
|
|
|
|
|
|||||
| Comprehensive loss |
(16,984 | ) | (5,167 | ) | ||||
| Comprehensive loss attributable to noncontrolling interests |
(12,443 | ) | (731 | ) | ||||
|
|
|
|
|
|||||
| Comprehensive loss attributable to Maravai Topco Holdings, LLC member |
$ | (4,541 | ) | $ | (4,436 | ) | ||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the consolidated financial statements
F-8
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF MEMBER’S EQUITY
(in thousands except unit amounts)
| Units | Amount | Contributed Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Noncontrolling Interests |
Total Member’s Equity |
||||||||||||||||||||||
| January 1, 2018 |
253,916,941 | $ | — | $ | 233,379 | $ | (33,765 | ) | $ | (98 | ) | $ | 15,428 | $ | 214,944 | |||||||||||||
| Distributions to members |
— | — | (52,056 | ) | — | — | — | (52,056 | ) | |||||||||||||||||||
| Repurchase of incentive units |
— | — | (9 | ) | — | — | — | (9 | ) | |||||||||||||||||||
| Unit-based compensation |
— | — | 1,495 | — | — | 626 | 2,121 | |||||||||||||||||||||
| Net loss |
— | — | — | (4,472 | ) | — | (12,443 | ) | (16,915 | ) | ||||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | — | (69 | ) | — | (69 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| December 31, 2018 |
253,916,941 | $ | — | $ | 182,809 | $ | (38,237 | ) | $ | (167 | ) | $ | 3,611 | $ | 148,016 | |||||||||||||
| Cumulative effect of adoption of ASC 606 |
— | — | — | 326 | — | — | 326 | |||||||||||||||||||||
| Repurchase of incentive units |
— | — | (227 | ) | — | — | — | (227 | ) | |||||||||||||||||||
| Unit-based compensation |
— | — | 1,328 | — | — | 351 | 1,679 | |||||||||||||||||||||
| Net loss |
— | — | — | (4,470 | ) | — | (731 | ) | (5,201 | ) | ||||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | — | 34 | — | 34 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| December 31, 2019 |
253,916,941 | $ | — | $ | 183,910 | $ | (42,381 | ) | $ | (133 | ) | $ | 3,231 | $ | 144,627 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements
F-9
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Year Ended December 31, | ||||||||
| 2018 | 2019 | |||||||
| Operating activities |
||||||||
| Net loss |
$ | (16,915 | ) | $ | (5,201 | ) | ||
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: |
||||||||
| Depreciation |
2,225 | 3,810 | ||||||
| Amortization of intangible assets |
20,122 | 20,274 | ||||||
| Provision of doubtful accounts |
(415 | ) | (152 | ) | ||||
| Amortization of deferred financing costs |
1,498 | 1,734 | ||||||
| Unit-based compensation |
2,121 | 1,679 | ||||||
| Loss on debt refinancing |
5,622 | — | ||||||
| Deferred income taxes |
317 | (1,159 | ) | |||||
| Change in estimated fair value of contingent consideration |
939 | 322 | ||||||
| Other |
141 | 518 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable |
(4,146 | ) | (1,888 | ) | ||||
| Inventory |
2,311 | 106 | ||||||
| Prepaid expenses and other current assets |
(696 | ) | (1,983 | ) | ||||
| Other non-current assets |
(570 | ) | — | |||||
| Accounts payable |
(871 | ) | 2,470 | |||||
| Accrued expenses and other current liabilities |
3,123 | 3,505 | ||||||
| Earn-out liability |
(14,547 | ) | — | |||||
| Other long-term liabilities |
354 | — | ||||||
| Deferred revenue |
(799 | ) | 80 | |||||
|
|
|
|
|
|||||
| Net cash (used in) provided by operating activities |
(186 | ) | 24,115 | |||||
|
|
|
|
|
|||||
| Investing activities |
||||||||
| Working capital adjustment for acquisition in prior year |
160 | — | ||||||
| Acquisition of patents |
(70 | ) | — | |||||
| Purchases of property and equipment |
(3,541 | ) | (17,148 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(3,451 | ) | (17,148 | ) | ||||
|
|
|
|
|
|||||
| Financing activities |
||||||||
| Distributions to members |
(52,056 | ) | — | |||||
| Proceeds from borrowings of long-term debt |
310,630 | — | ||||||
| Financing costs incurred |
(6,711 | ) | — | |||||
| Repurchase of vested incentive units |
(9 | ) | (227 | ) | ||||
| Principal repayments of long-term debt |
(254,987 | ) | (2,500 | ) | ||||
| Payment of contingent consideration |
(5,819 | ) | (1,300 | ) | ||||
| Payments made on facility financing lease obligation and capital lease |
(215 | ) | (140 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in financing activities |
(9,167 | ) | (4,167 | ) | ||||
| Effects of exchange rate changes on cash |
(69 | ) | 34 | |||||
|
|
|
|
|
|||||
| Net (decrease) increase in cash, and restricted cash |
(12,873 | ) | 2,834 | |||||
| Cash, beginning of period |
34,739 | 21,866 | ||||||
|
|
|
|
|
|||||
| Cash, end of period |
$ | 21,866 | $ | 24,700 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow information |
||||||||
| Cash paid for interest |
$ | 25,678 | $ | 28,728 | ||||
|
|
|
|
|
|||||
| Cash paid for income taxes |
$ | 97 | $ | 802 | ||||
|
|
|
|
|
|||||
| Supplemental disclosures of non-cash investing and financing activities |
|
|||||||
| Property and equipment included in accounts payable and accrued expenses |
$ | 196 | $ | 2,765 | ||||
|
|
|
|
|
|||||
| Financing cost deducted from loan proceeds |
$ | 3,800 | $ | — | ||||
|
|
|
|
|
|||||
| Building and improvements capitalized under lease financing transaction |
$ | 15,374 | $ | 51,200 | ||||
|
|
|
|
|
|||||
| Property and equipment under new capital lease |
$ | 87 | $ | 15 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the consolidated financial statements
F-10
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Significant Accounting Policies
Organization and Description of Business
Maravai Topco Holdings, LLC (“Maravai,” “we,” “our” or the “Company”) is a holding company focused on building a transformative life sciences products company by acquiring businesses and accelerating their growth through capital infusions and industry expertise. The Company was organized as a Delaware limited liability company (“LLC”) on July 27, 2018, as a wholly-owned subsidiary of Maravai Life Sciences Holdings, LLC (“MLSH 1”). On this date, the Company acquired all of the equity interests of a subsidiary of MLSH 1 that held all of the operating subsidiaries of MLSH 1. This acquisition was a transfer between entities under common control in a manner consistent with the pooling-of-interest method of accounting. These consolidated and combined financial statements for the year-ended December 31, 2018 give effect to this transaction as if it had occurred at the beginning of the period presented. As this entity was considered to be the predecessor entity under common control and accordingly, the accompanying combined statement of operations, comprehensive loss, member’s equity, and cash flows present the results of operations of MLSH 1 beginning on January 1, 2018.
The Company is headquartered in San Diego, California and has three principal businesses: Nucleic Acid Production, Biologics Safety Testing, and Protein Detection. Our Nucleic Acid Production business manufactures and sells products used in the fields of gene therapy, nucleoside chemistry, oligonucleotide therapy and molecular diagnostics, including reagents used in the chemical synthesis, modification, labelling and purification of deoxyribonucleic acid (“DNA”) and ribonucleic acid (“RNA). Our Biologics Safety Testing business sells highly specialized analytical products for use in biologic manufacturing process development, including custom product-specific development antibody and assay development services. Our Protein Detection business sells innovative labeling and detection reagents for researchers in immunohistochemistry.
In October 2016, the Company acquired a controlling interest in MLSC Holdings, LLC (“MLSC”), the parent entity of one of our biologics safety testing operating companies, by purchasing approximately 70% of the equity interests of MLSC with the remaining 30% being recorded as noncontrolling interests in the consolidated financial statements of the Company.
Basis of Presentation
These consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The consolidated financial statements include the accounts of the Company and its wholly-owned and majority-owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Unit Split
On November 11, 2020, the Company’s member approved an amendment to the Company’s limited liability company agreement to increase the authorized common units from 1,000 to 253,916,941 and effect a 253,916.941-for-1 split of its common units. All of the Company’s unit and per unit information included in the accompanying consolidated financial statements has been adjusted to reflect the split.
Use of Estimates
The preparation of consolidated financial statements in accordance with GAAP requires the Company to make estimates and assumptions that affect the reported amounts in the consolidated financial statements and accompanying notes. These estimates form the basis for judgments the Company makes about the carrying values of assets and liabilities that are not readily apparent from other sources. The Company bases its estimates
F-11
and judgments on historical experience and on various other assumptions that the Company believes are reasonable under the circumstances. These estimates are based on management’s knowledge about current events and expectations about actions the Company may undertake in the future. Significant estimates include, but are not limited to, revenue recognition, the net realizable value of inventory, expected future cash flows including growth rates, discount rates, terminal values and other assumptions and estimates used to evaluate the recoverability of long-lived assets, estimated fair values of intangible assets and goodwill, amortization methods and periods, valuation of intangible assets, the fair value of leased buildings and other assumptions associated with lease financing transactions, the estimated fair value of our long-term debt, unit-based compensation, the valuation of our and MLSH 1’s incentive units, allowance for doubtful accounts, and accounting for income taxes. Actual results could differ materially from those estimates.
Revenue Recognition
The Company adopted the requirements of Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers (“ASC 606”), effective January 1, 2019 using the modified retrospective method. Under the modified retrospective method, this guidance is applied to those contracts that were not completed as of January 1, 2019, with no restatement of contracts that were commenced and completed within fiscal years prior to January 1, 2019, and the prior period comparable financial information continues to be presented under the guidance of ASC 605, Revenue Recognition (“ASC 605”). The adoption of ASC 606 resulted in a cumulative effect adjustment of $0.3 million to reduce the opening accumulated deficit as of January 1, 2019. This adjustment primarily related to over-time recognition of revenue and associated costs for certain custom products for which revenue had previously been deferred and recognized at a point in time.
The Company generates revenue from the sale of products and services and the performance of services in the fields of nucleic acid production, biologics safety testing, and protein detection. Under ASC 606, revenue is recognized when control of promised goods or services is transferred to a customer in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To determine revenue recognition for its arrangements with customers, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. The majority of the Company’s contracts include only one performance obligation. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is defined as the unit of account for revenue recognition under ASC 606. The Company also recognizes revenue from other contracts that may include a combination of products and services, the provision of solely services, or from license fee arrangements which may be associated with the delivery of product. Where there is a combination of products and services, the Company accounts for the promises as individual performance obligations if they are concluded to be distinct. Performance obligations are considered distinct if they are both capable of being distinct and distinct within the context of the contract. In determining whether performance obligations meet the criteria for being distinct, the Company considers a number of factors, such as the degree of interrelation and interdependence between obligations, and whether or not the good or service significantly modifies or transforms another good or service in the contract. As a practical expedient, we do not adjust the transaction price for the effects of a significant financing component if, at contract inception, the period between customer payment and the transfer of goods or services is expected to be one year or less. Contracts with customers are evaluated on a contract-by-contract basis as contracts may include multiple types of goods and services as described below.
Nucleic Acid Production
Nucleic acid production revenue is generated from the manufacture and sale of highly modified, complex nucleic acids products to support the needs of our of customers’ research, therapeutic and vaccine programs. The primary offering of products include; CleanCap®, mRNA, and specialized oligonucleotides. Contracts typically consist of a single performance obligation. We also sell nucleic acid products for labeling and detecting proteins in cells and tissue samples research. The Company recognizes revenue from these products in the period in which
F-12
the performance obligation is satisfied by transferring control to the customer. Revenue for nucleic acid catalog products is recognized at a single point in time, generally upon shipment to the customer. Revenue for contracts for certain custom nucleic acid products, with an enforceable right to payment and a reasonable margin for work performed to date, is recognized over time, based on a cost-to-cost input method over the manufacturing period.
Biologics Safety Testing
The Company’s biologics safety testing revenue is associated with the sale of bioprocess impurity detection kit products. We also enter into contracts that include custom antibody development, assay development and antibody affinity extraction services. These products and services enable the detection of impurities and contaminants that occur in the manufacturing of biologic drugs and other therapeutics. The Company recognizes revenue from the sale of bioprocess impurity detection kits in the period in which the performance obligation is satisfied by transferring control to the customer. Custom antibody development contracts consist of a single performance obligation, typically with an enforceable right to payment and a reasonable margin for work performed to date. Revenue is recognized over time based on a cost-to-cost input method over the contract term. Where an enforceable right to payment does not exist, revenue is recognized at a point in time when control is transferred to the customer. Assay development service contracts consist of a single performance obligation, revenue is recognized at a point in time when a successful antigen test and report is provided to the customer. Affinity extraction services, which generally occur over a short period of time, consist of a single performance obligation to perform the extraction service and provide a summary report to the customer. Revenue is recognized either over time or at a point in time depending on contractual payment terms with the customer.
The Company also has certain licensing and royalty arrangements with an immaterial amount of revenue.
Protein Detection
The Company also manufactures and sells protein labeling and detection reagents to customers that are used for basic research and development. The contracts to sell these catalog products consist of a single performance obligation to deliver the reagent products. Revenue from these contracts is recognized at a point in time, generally upon shipment of the final product to the customer.
We recognize royalty revenue related to certain out-licensing and royalty arrangements in the period the sales or usage occur using third-party evidence to estimate the amount to be recorded. To date this revenue has not been material to the consolidated financial statements.
The Company has elected the practical exemption to not disclose the unfulfilled performance obligations for contracts with an original length of one year or less. The Company has no material unfulfilled performance obligations for contracts with an original length greater than one year at December 31, 2019.
The Company accepts returns only if the products do not meet customer specifications and historically, the Company’s volume of product returns has not been significant. Further, no warranties are provided for promised goods and services other than assurance type warranties.
Revenue for an individual contract is recognized at the related transaction price, which is the amount the Company expects to be entitled to in exchange for transferring the products and/or services. The transaction price for product sales is calculated at the contracted product selling price. The transaction price for a contract with multiple performance obligations is allocated to the separate performance obligations on a relative standalone selling price basis. Standalone selling prices for products are determined based on the prices charged to customers, which are directly observable. Standalone selling price of services are mostly based on time and materials. Generally, payments from customers are due when goods and services are transferred. As most contracts contain a single performance obligation, the transaction price is representative of the standalone selling price charged to customers. Revenue is recognized only to the extent that it is probable that a significant reversal of the cumulative amount recognized will not occur in future periods. Since the adoption of ASC 606, variable consideration has not been material.
F-13
Sales taxes
Sales taxes collected by the Company are not included in the transaction price as revenue they are ultimately remitted to a governmental authority.
Shipping and handling costs
The Company has elected to account for shipping and handling activities related to contracts with customers as costs to fulfill the promise to transfer the associated products. Accordingly, revenue for shipping and handling is recognized at the same time that the related product revenue is recognized.
Contract costs
The Company recognizes the incremental costs of obtaining contracts as an expense when incurred when the amortization period of the assets that otherwise would have been recognized is one year or less. These costs are included in sales and marketing and general and administrative expenses. The costs to fulfill the contracts are determined to be immaterial and are recognized as an expense when incurred.
Contract balances
Contract assets are generated when contractual billing schedules differ from revenue recognition timing and the Company records contract receivable when it has an unconditional right to consideration. No contract assets balance was recorded as of January 1, 2019 and as of December 31, 2019, contract assets, which are included in prepaid and other current assets, totaled $0.8 million.
Contract liabilities are recorded when cash payments are received or due in advance of performance. Contract liabilities consist of customer deposits, which are included in accrued expenses, and deferred revenue, where the Company has unsatisfied performance obligations. As of January 1, 2019 and December 31, 2019, the contract liabilities were $0.5 million and $1.0 million, respectively, with the contract liabilities at December 31, 2019 expected to be recognized into revenue in the year ending December 31, 2020.
Disaggregation of Revenue
The following tables summarize the revenue by segment and region for the years ended December 31, 2018 and December 31, 2019, respectively (in thousands):
| For the year ended December 31, 2018 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Total | ||||||||||||
| North America |
$ | 44,883 | $ | 14,900 | $ | 14,393 | $ | 74,176 | ||||||||
| Europe, the Middle East and Africa |
9,880 | 11,443 | 6,522 | 27,845 | ||||||||||||
| Asia Pacific |
5,249 | 11,928 | 4,097 | 21,274 | ||||||||||||
| Latin and Central America |
45 | 221 | 272 | 538 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
$ | 60,057 | $ | 38,492 | $ | 25,284 | $ | 123,833 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| For the year ended December 31, 2019 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Total | ||||||||||||
| North America |
$ | 49,757 | $ | 18,984 | $ | 15,284 | $ | 84,025 | ||||||||
| Europe, the Middle East and Africa |
15,975 | 12,102 | 6,805 | 34,882 | ||||||||||||
| Asia Pacific |
6,843 | 12,964 | 3,784 | 23,591 | ||||||||||||
| Latin and Central America |
27 | 366 | 249 | 642 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
$ | 72,602 | $ | 44,416 | $ | 26,122 | $ | 143,140 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-14
The following table provides a disaggregation of revenue for the year ended December 31, 2019 based on the pattern of revenue recognition (in thousands):
| 2019 | ||||
| Revenue recognized at a point in time |
$ | 133,091 | ||
| Revenue recognized over time |
10,049 | |||
|
|
|
|||
| Total revenue |
$ | 143,140 | ||
|
|
|
|||
Prior to January 1, 2019, revenue from the sale of products and services was recognized when all of the following conditions per ASC 605 were met: (1) there was persuasive evidence of an arrangement; (2) the product had been delivered to the customer; (3) the collection of the fees was reasonably assured; and (4) the amount of fees to be paid by the customer was fixed or determinable.
When an arrangement involved multiple elements, the multiple elements, referred to as deliverables, were evaluated to determine whether they represent separate units of accounting in accordance with ASC 605-25, Revenue Recognition—Multiple-Element Arrangements. The Company performed this evaluation at the inception of an arrangement and as each item was delivered in the arrangement. Generally, the Company accounted for a deliverable separately if the delivered item has stand alone value to the customer and delivery or performance of the undelivered item or service was probable and substantially in the Company’s control.
When multiple elements could be separated into separate units of accounting, arrangement consideration was allocated at the inception of the arrangement, based on each unit’s relative selling price, and recognized based on the method most appropriate for that unit.
Product sales
Revenue for manufacturing of products was recognized upon the delivery of the products in accordance with the terms of the contract, which specify transfer of title and risk of loss. Payments received from customers in advance of manufacturing their products was recorded as deferred revenue until the products were delivered.
Service revenue
The Company also enters into custom antibody and assay development contracts with customers. The Company performs a number of acts under these contracts for which the pattern of performance cannot be discerned and therefore the Company recognizes service revenue on a straight-line basis over the contractual term or expected term of the arrangement, whichever is longer. Payments received in advance of performing these services was recognized as deferred revenue. Revenue recognized at any point in time is limited to cash received and amounts contractually due.
Shipping and Handling Costs
Shipping and handling costs, which are charged to customers, are included in revenue. Shipping and handling charges included in revenue were approximately $2.8 million and $3.2 million and for the years ended December 31, 2018 and 2019, respectively. Freight and supplies costs directly associated with shipping products to customers are included as a component of cost of revenue.
Research and Development
Research and development (“R&D”) expenses include personnel costs, including salaries, benefits and unit-based compensation for laboratory personnel, and costs of supplies. R&D costs are expensed as incurred. Payments made prior to the receipt of goods or services to be used in R&D are recognized as prepaid assets until the goods are received or services are rendered.
F-15
Advertising Costs
The Company expenses advertising costs as incurred. Advertising costs incurred were approximately $0.6 million and $1.1 million during the years ended December 31, 2018 and 2019, respectively.
Unit-Based Compensation
The Company’s parent, MLSH 1, grants unit-based awards to certain executives of the Company in the form of non-vested units. Our controlled subsidiary, MLSC, grants unit-based awards only to certain employees of its subsidiaries (collectively, the “Incentive Units”). All awards of Incentive Units are measured based on the fair value of the award on the date of grant. The Company recognizes compensation expense for MLSH 1 awards in its consolidated financial statements as MLSH 1 is considered to be the economic interest holder in the Company. Compensation expense for the Incentive Units is recognized over their requisite service period. Forfeitures are recognized when they occur. These Incentive Units are subject to service, market and performance conditions. For Incentive Units subject to performance conditions, the Company evaluates the probability of achieving each performance condition at each reporting date and recognizes expense over the requisite service period when it is deemed probable that a performance condition will be met using the accelerated attribution method over the requisite service period. For Incentive Units that remain subject to performance conditions at December 31, 2018 and 2019, the Company concluded that it was not yet probable that the performance conditions would be met. Accordingly, the Company has not recognized any compensation expense in the accompanying consolidated statements of operations and comprehensive loss for Incentive Units that include a performance condition.
The grant date fair value of Incentive Unit awards has been determined by the Company’s Board of Directors with the assistance of management and an independent third-party valuation specialist. The grant date fair value of Incentive Units was determined first by estimating an aggregate equity value using a weighting of discounted cash flows, comparable public companies, and comparable-transactions valuation methodologies. An Option-Pricing Method, which utilizes certain assumptions including volatility, time to liquidation, a risk-free interest rate, and an assumption for a discount for lack of marketability, was then used to allocate the total equity value of the Company to the different classes of equity according to their rights and preferences. In determining the fair value of the Incentive Units, the methodologies used to estimate the enterprise value of the Company were performed using methodologies, approaches, and assumptions consistent with the American Institute of Certified Public Accountants Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation (“AICPA Accounting and Valuation Guide”).
Income Taxes
The Company’s wholly-owned U.S. subsidiary, Maravai Life Sciences, Inc. (“Maravai Inc.”) and its subsidiaries, are taxpaying entities in the U.S., Canada, and the U.K. Accordingly, the Company provides current and deferred income taxes for these entities. The Company and its other subsidiaries are treated as pass-through entities for federal and state income tax purposes and are not subject to income tax as the LLC member is responsible for the tax consequences of its proportionate share of the pass-through income or loss. As such, the Company’s tax provision consists solely of the activities of Maravai Inc. and its subsidiaries, which is taxed as a corporation for federal and state income tax purposes.
The Company’s taxable subsidiaries account for income taxes using the asset and liability method, under which deferred tax liabilities and assets are recognized for the expected future tax consequences of temporary differences between the consolidated financial statement carrying amounts and the tax basis of assets and liabilities and net operating loss (“NOL”) and tax credit carryforwards. Deferred tax assets and liabilities are classified as noncurrent on the consolidated balance sheet. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. The Company uses a recognition threshold and measurement attribute for the consolidated financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. A tax position is recognized when it is more likely than not that the
F-16
tax position will be sustained upon examination, including the resolution of any related appeals or litigation. A tax position that meets the more-likely-than-not recognition threshold is measured at the largest amount of benefit that is greater than a 50% likelihood of being realized upon ultimate settlement with a taxing authority. Interest and penalties related to unrecognized tax benefits are recognized in benefit from income taxes in the accompanying consolidated statements of operations and comprehensive loss. No such interest and penalties were recognized for any period presented.
The Tax Cuts and Jobs Act of 2017 (“2017 Tax Act”) created a Global Intangible Low Taxed Income (“GILTI”) provision which requires companies to include in its U.S. income tax return foreign subsidiary earnings in excess of an allowable return on the foreign subsidiary’s tangible assets. The Company has elected to account for GILTI tax as a component of tax expense in the period in which it is incurred. For the years ended December 31, 2018 and 2019, the Company did not recognize income related to its foreign subsidiaries.
In May 2018, the FASB issued Accounting Standards Update (“ASU”) 2018-05—Income Taxes (Topic 740), Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 118 (SEC Update). The amendment represents changes to certain SEC material in Topic 740 for the income tax accounting implications of the 2017 Tax Act. The ASU was effective upon issuance. As of December 22, 2018, the Company had completed its assessment and recorded an insignificant adjustment to the provisional amounts initially recognized within the one-year period provided by this ASU.
Noncontrolling Interests
Noncontrolling interests represent the portion of profit or loss, net assets and comprehensive loss that is not allocable to the member of Maravai. Noncontrolling interests arise from the Company’s majority-owned subsidiary, MLSC, in which the Company holds a 70% ownership interest. MLSC is not liable, solely by reason of being a member, for the debts, obligations, or liabilities of the Company whether arising in contract or tort; under a judgment, decree, or order of a court; or otherwise. MLSC net income or loss is attributed to the noncontrolling interests using an attribution method, similar to the hypothetical liquidation at book value method, based on the distribution provisions of the MLSC Amended and Restated Limited Liability Company Agreement (“MLSC LLC Agreement”).
During the year ended December 31, 2018, $52.0 million of capital distributions were made to the Class A unit holders of MLSC. The 2018 distribution was treated as a preferred return of capital per the terms of the MLSC LLC Agreement, which will result in a reduction of future amounts that must be allocated to the Class A unit holders of MLSC, as controlling interest holders in MLSC, upon a liquidation of MLSC. No distributions were made to the Class A unit holders of MLSC during the year ended December 31, 2019.
Segment Information
The Company operates in three reportable segments. Operating segments are defined as components of an enterprise about which separate financial information is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and assessing performance. The Company’s chief operating decision maker (“CODM”), its Chief Executive Officer, allocates resources and assesses performance based upon discrete financial information at the segment level. Substantially all of our long-lived assets are located in the United States.
Cash
Cash consists of deposits held at financial institutions.
F-17
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable primarily consist of amounts due from customers for product sales and services. Estimated allowances for doubtful accounts are provided for based on an evaluation of potential uncollectible accounts. The Company evaluates accounts receivable to determine collectability. Judgments and estimates are involved in performing this evaluation, which are based on the Company’s assessment of a customer’s ability to pay, credit quality of the customer, age of the receivable balance and current economic conditions. As of December 31, 2018 and 2019, the allowance for doubtful accounts was approximately $0.3 million and $0.1 million, respectively. Write-offs of accounts receivable and recoveries were not significant during either 2018 or 2019.
To manage credit risk certain Company subsidiaries require select customers to prepay for product prior to shipment. Such prepayments approximated $40,000 and $0.2 million as of December 31, 2018 and 2019, respectively, and were recorded within deferred revenue and subsequently recognized as revenue upon shipment.
Inventory
Inventories consist of raw materials, work in process and finished goods. Inventories are stated at the lower of cost (weighted average cost) or net realizable value. Inventory costs include materials, direct labor and manufacturing overhead, which are related to the purchase or production of inventories. The Company regularly monitors for excess and obsolete inventory based on its estimates of expected sales volumes, production capacity and expiration of raw materials, work-in-process and finished products excess and obsolete inventories and reduces the carrying value of inventory accordingly. The Company writes down inventory that has become obsolete, inventory that has a cost basis in excess of its expected net realizable value, and inventory in excess of expected manufacturing requirements. Any write-downs of inventories are charged to cost of revenue.
A change in the estimated timing or amount of demand for the Company’s products could result in reduction to the recorded value of inventory quantities on hand. Any significant unanticipated changes in demand or unexpected quality failures could have a significant impact on the value of inventory and reported operating results. During all periods presented in the accompanying consolidated financial statements, there have been no material adjustments related to a revised estimate of our inventory valuations.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the following estimated useful lives:
| Assets |
Useful Lives | |||
| Buildings |
20-35 years | |||
| Building improvements |
5-15 years | |||
| Furniture, fixtures, equipment and software |
3-11 years | |||
Leasehold improvements are amortized over the shorter of the related lease term or useful life.
Maintenance and repairs are charged to operations when incurred, while betterments or renewals are capitalized. When property and equipment are sold or otherwise disposed of, the asset account and related accumulated depreciation account are relieved, and any gain or loss is included in the results of operations.
As of December 31, 2018, property and equipment included a leased building which is recorded as construction in process during the period of construction at its fair value plus the cost of improvements incurred during the construction period. As of December 31, 2019, the construction was completed. As a result, the Company recorded constructions costs as an asset and construction costs incurred by the landlord as a financing
F-18
obligation on its consolidated balance sheet (Notes 5 and 6). The leased building is being depreciated over the lease term to a residual value that will approximate the remaining lease financing obligation at the end of the lease.
Internal-Use Software Costs
The Company capitalizes costs for acquired or developed software for internal use. Costs related to preliminary project activities and post implementation activities are expensed as incurred. Once it is probable the project will be completed, and the software will be used to perform the function intended, internal and external costs, if direct and incremental, are capitalized until the application is substantially complete and ready for use. Capitalized costs are included in property and equipment within furniture, fixtures, equipment, and software. The Company capitalized approximately $0.4 million and $0.7 million of software development costs in each of the years ended December 31, 2018 and 2019, respectively, and recognized amortization expense of $73,000 and $0.2 million for the years ended December 31, 2018 and 2019, respectively.
Goodwill
Goodwill represents the excess of consideration transferred over the estimated fair value of assets acquired and liabilities assumed in a business combination. The Company conducts a goodwill impairment analysis at least annually and more frequently if changes in facts and circumstances indicate that the fair value of the Company’s reporting units may be less than carrying amount. The Company has three reporting units. For the years ended December 31, 2018 and 2019, the Company elected the qualitative assessment for two of its reporting units and a quantitative impairment test for its remaining reporting unit associated with its protein detection business. The qualitative impairment test was elected for these two reporting units because of the growth in revenue and cashflows in excess of our initial projections and quantitative impairment test was elected for the third reporting unit as a result of lowering forecasted growth projections. As a result of the 2018 and 2019 qualitative and quantitative assessments, the Company concluded that goodwill was not impaired at both December 31, 2018 and 2019.
Intangible Assets
The Company’s finite-lived intangible assets represent purchased intangible assets and primarily consist of trade names, customer relationships, patents, and developed technology. Certain criteria are used in determining whether intangible assets acquired in a business combination must be recognized and reported separately. Finite-lived intangible assets are initially recognized at fair value, are subject to amortization and are subsequently stated at amortized cost. The Company’s finite-lived intangible assets are amortized using a method that reflects the pattern in which the economic benefits of the intangible assets are consumed or otherwise used. If that pattern cannot be reliably determined, the intangible assets are amortized using the straight-line method over their estimated useful lives and are tested for impairment along with other long-lived assets. Amortization related to patents and developed technology is allocated to cost of revenue whereas amortization associated with trade names and customer relationships is allocated to selling, general and administrative expenses.
Impairment of Long-Lived and Intangible Assets
The Company periodically reviews long-lived assets, including property and equipment and finite-lived intangible assets, to determine whether current events or circumstances indicate that such carrying amounts may not be recoverable. If such facts or circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets is compared to the carrying value the assets to determine whether impairment exists. If the assets are determined to be impaired, the loss is measured based on the difference between the fair value and carrying value of the assets. No impairment loss was recognized for long-lived or finite-lived intangible assets during the years ended either December 31, 2018 or 2019.
F-19
Contingent Consideration
Contingent consideration relates to the potential payment for an acquisition that is contingent upon the achievement of the acquired entity meeting certain performance milestones. Contingent consideration resulting from the acquisition of a business is recorded at fair value on the acquisition date. Such contingent consideration is re-measured to its estimated fair value at each reporting date with the change in fair value recognized as an operating expense in the Company’s consolidated statement of operations. Subsequent changes in the fair value of the contingent consideration are classified as an adjustment to cash flows from operating activities in the consolidated statement of cash flows because the change in fair value was is an input in determining net loss. Cash paid in settlement of contingent consideration liabilities are classified as cash flows from financing activities up to the acquisition date fair value with any excess classified as cash flows from operating activities.
Changes in the fair value of contingent consideration liabilities associated with the acquisition of a business can result from updates to assumptions such as the expected timing or probability of achieving customer related performance targets, specified sales milestones, changes in projected revenue or changes in discount rates. Significant judgment is used in determining those assumptions as of the acquisition date and for each subsequent reporting period. Therefore, any changes in the fair value will impact the Company’s results of operations in such reporting period thereby resulting in potential variability in the Company’s operating results until such contingencies are resolved.
Debt Issuance Costs
Costs incurred in connection with obtaining new debt financing are deferred and amortized over the life of the related financing. If such financing is settled or replaced prior to maturity with debt instruments that have substantially different terms, the settlement is treated as an extinguishment and the unamortized costs are charged to gain or loss on extinguishment of debt. If such financing is settled or replaced with debt instruments from the same lender that do not have substantially different terms, the new debt agreement is accounted for as a modification for the prior debt agreement and the unamortized costs remain capitalized, the new original issuance discount costs are capitalized, and any new third-party costs are charged to expense. Deferred costs are recognized as a direct reduction in the carrying amount of the debt instrument on the consolidated balance sheets and are amortized to interest expense over the term of the related debt using the effective interest method.
Foreign Currency
The Company translates the assets and liabilities of its non-U.S. dollar denominated functional currency subsidiaries into U.S. dollars using exchange rates in effect at the end of each period. Revenue and expenses for its foreign subsidiaries are translated using rates that approximate those in effect during the period. Translation gains and losses are recognized in accumulated other comprehensive loss within member’s equity on the consolidated balance sheets. Foreign currency transaction gains and losses are included in net loss for the period. Foreign currency loss have not been material for the years ended December 31, 2018 and 2019.
Accumulated Other Comprehensive Loss
Comprehensive loss and its components encompass all changes in member’s equity other than those with its members. Comprehensive loss for the Company consists of foreign currency translation adjustments. There were no reclassifications out of accumulated other comprehensive loss during the periods presented.
Fair Value of Financial Instruments
The Company defines fair value as the amount that would be received to sell an asset, or paid to transfer a liability, in an orderly transaction between market participants at the measurement date. The Company follows accounting guidance that has a three-level hierarchy for fair value measurements based upon the transparency of
F-20
inputs to the valuation of the asset or liability as of the measurement date. Instruments with readily available actively quoted prices, or for which fair value can be measured from actively quoted prices in an orderly market, will generally have a higher degree of market price transparency and a lesser degree of judgment used in measuring fair value. The three levels of the hierarchy are defined as follows:
Level 1—Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets;
Level 2—Include other inputs that are directly or indirectly observable in the marketplace; and
Level 3—Unobservable inputs which are supported by little or no market activity.
As of December 31, 2018 and 2019, the carrying value of current assets and liabilities approximates fair value due to the short maturities of these instruments. The fair values of the Company’s long-term debt approximate carrying value, excluding the effect of unamortized debt discount, as they are based on borrowing rates currently available to the Company for debt with similar terms and maturities (Level 2 inputs).
During 2017, the Company entered into two interest rate cap agreements to manage a portion of its variable interest rate risk on its amended credit agreement borrowings. During 2018, in connection with the Company’s debt refinancing, the Company entered into two interest rate cap agreements bringing its total to four agreements as of both December 31, 2018 and 2019. The contracts entitle the Company to receive from the counterparty at specified dates the amount, if any, by which a specified market rate exceeds the cap strike interest rate, applied to the contracts notional principal amount of approximately $262.0 million. No principal payments are exchanged. The interest rate cap agreements have not been designated as a hedging relationship and are recognized on the consolidated balance sheet at fair value within non-current assets with changes in fair value recognized in the consolidated statements of operations and comprehensive loss. The fair value of the interest rate caps as of December 31, 2018 and 2019 were insignificant.
Leases, Deferred Rent, and Lease Facility Financing Obligation
The Company rents its office space and facilities under non-cancelable operating lease agreements and recognizes the related rent expense on a straight-line basis over the term of the lease. The Company’s lease agreements contain rent holidays, scheduled rent increases, and renewal options. Rent holidays and scheduled rent increases are included in the determination of rent expense to be recorded ratably over the lease term. The Company does not assume renewals in its determination of the lease term unless they are deemed to be reasonably assured at the inception of the lease. The Company begins recognizing rent expense on the date that it obtains the legal right to use and control the leased space. Deferred rent consists of the difference between cash payments and the recognition of rent expense on a straight-line basis for the buildings the Company occupies.
In certain arrangements, the Company is involved in the construction of improvements to buildings it is leasing. To the extent the Company is involved with the structural improvements of the construction project or takes construction risk, the Company is considered to be the owner of the building and related improvements for accounting purposes during the construction period. The Company records the fair value of the building and related landlord and lessee funded improvements subject to the lease within property and equipment on the consolidated balance sheet. The Company also records a corresponding construction payable obligation on its consolidated balance sheet representing the amounts financed by the lessor for the building and lessor financed improvements. Once a construction project is complete, the Company considers the requirements for sale-leaseback accounting treatment. If the Company concludes the arrangement does not qualify for sale-leaseback accounting treatment, the building and related improvements remain on the Company’s consolidated balance sheet and are subject to depreciation and assessment of impairment.
For such arrangements, at both pre and post the construction period, the Company bifurcates its lease payments into a portion allocated to the building and a portion allocated to the parcel of land on which the
F-21
building has been built considering their respective fair values. The land lease portion of the lease payments allocated to the land is treated for accounting purposes as operating lease payments, and therefore is recorded as rent expense in the consolidated statements of operations. The portion of the lease payments allocated to the building is further bifurcated into a portion allocated to interest expense and a portion allocated to reduce the lease financing obligation. The interest rate used for the lease financing obligation represents the Company’s estimated incremental borrowing rate at the inception of the lease, adjusted to reduce any built-in loss.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and accounts receivable. The Company maintains the majority of its cash balances at multiple financial institutions that management believes are of high-credit quality and financially stable. Cash is deposited with major financial institutions in excess of Federal Deposit Insurance Corporation (“FDIC”) insurance limits. At December 31, 2018 and 2019, the Company had approximately $20.5 million and $23.6 million in six major financial institutions, respectively, in excess of FDIC insurance limitations. The Company provides credit, in the normal course of business, to international and domestic distributors and customers, which are geographically dispersed. The Company attempts to limit its credit risk by performing ongoing credit evaluations of its customers and maintaining adequate allowances for potential credit losses. For the year ended December 31, 2018, no customer represented more than 10% of total consolidated revenue. For the year ended December 31, 2019, Thermo Fisher Scientific Inc., one of the global leaders in serving life sciences represented 10% of our revenue. The Company sells multiple products to Thermo Fisher Scientific Inc. across all three of the business segments. For the year ended December 31, 2018, one single customer represented 13% of total consolidated accounts receivable. For the year ended December 31, 2019, two customers accounted for 11% and 10% of total consolidated accounts receivable.
Net Loss per Common Unit Attributable to the Member
Basic net loss per common unit attributable to the member of Maravai is calculated by dividing the net loss, adjusted for the Company’s noncontrolling interests by the weighted-average number of common units outstanding during the period. The noncontrolling interests is calculated pursuant to the terms of the MLSC LLC Agreement, the Company’s majority-owned subsidiary, on a fully-distributed basis, taking into account the various classes of equity of MLSC, including the cumulative yields on MLSC’s preferred units. Diluted net loss per common unit attributable to the member of Maravai is computed by using the weighted-average number of common units outstanding during the period and the potential dilutive common unit equivalents as determined under the two-class method. In periods in which the Company reports a net loss attributable to the member of Maravai, diluted net loss per common unit is the same as basic net loss per common unit attributable to the member of Maravai since dilutive common units are not assumed to have been issued if their effect is anti-dilutive. The Company reported a net loss attributable to the member of Maravai for the years ended December 31, 2018 and 2019.
Emerging Growth Company Status
The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these consolidated financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
F-22
Recently Adopted Accounting Pronouncements
In August 2016, the Financial Accounting Standards Board (“FASB”) issued ASU 2016-15, Statement of Cash Flows (Topic 230) provides guidance on eight specific cash flow issues, thereby reducing the diversity in practice in how certain transactions are classified in the statement of cash flows. The amendments in ASU 2016-15 were effective for annual reporting periods beginning after December 15, 2018. The Company’s adoption of this standard did not have an impact on the consolidated financial statements.
In October 2016, the FASB issued ASU 2016-16, Income Taxes—Intra-Entity Transfers of Assets Other Than Inventory. ASU 2016-16 requires entities to recognize income tax consequences of an intra-entity transfer of an asset other than inventory when the transfer occurs. The amendments in ASU 2016-16 are effective for annual reporting periods beginning after December 15, 2018 and required a modified retrospective method of adoption. The Company’s adoption of this standard did not have an impact on the consolidated financial statements.
Recent Issued Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, Leases (“Topic 842”), which supersedes the guidance in ASC 840, Leases. The new standard, as amended by subsequent ASUs on Topic 842 and recent extensions issued by the FASB in response to COVID-19, requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months regardless of their classification. Leases with a term of 12 months or less will be accounted for similar to existing guidance for operating leases today. As a result of the Company having elected the extended transition period for complying with new or revised accounting standards pursuant to Section 107(b) of the JOBS Act, and assuming the Company continues to be considered an Emerging Growth Company, Topic 842 will be effective for the Company on January 1, 2022. The Company has not yet determined the full effects of Topic 842 on its consolidated financial statements but does expect that it will result in a substantial increase in its long-term assets and liabilities and enhanced disclosures.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments which has been subsequently amended (“ASU 2016-13”). ASU 2016-13 revises the measurement of credit losses for most financial instruments measured at amortized cost, including trade receivables, from an incurred loss methodology to an expected loss methodology which results in earlier recognition of credit losses. Under the incurred loss model, a loss is not recognized until it is probable that the loss-causing event has already occurred. The new standard introduces a forward-looking expected credit loss model that requires an estimate of the expected credit losses over the life of the instrument by considering all relevant information including historical experience, current conditions, and reasonable and supportable forecasts that affect collectability. In addition, this standard also modifies the impairment model for available-for-sale debt securities, which are measured at fair value, by eliminating the consideration for the length of time fair value has been less than amortized cost when assessing credit loss for a debt security and provides for reversals of credit losses through income upon credit improvement. ASU 2016-13, is effective for the Company’s January 1, 2023, with early adoption permitted. The Company is currently assessing the impact of adopting this standard on its consolidated financial statements and disclosures.
In June 2018, the FASB issued ASU No. 2018-07, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting. ASU 2018-07 simplifies the accounting for share-based payment transactions in which a grantor acquires goods or services to be used or consumed in operations from a nonemployee. This standard is effective for annual periods beginning after December 15, 2019. The Company does not anticipate the adoption of this standard to have a material impact on its consolidated financial statements.
F-23
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820)—Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”). removed the following disclosure requirements: (1) the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy; (2) the policy for timing of transfers between levels; and (3) the valuation processes for Level 3 fair value measurements. Additionally, this update added the following disclosure requirements: (1) the changes in unrealized gains and losses for the period included in other comprehensive income and loss for recurring Level 3 fair value measurements held at the end of the reporting period; (2) the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. For certain unobservable inputs, an entity may disclose other quantitative information (such as the median or arithmetic average) in lieu of the weighted average if the entity determines that other quantitative information would be a more reasonable and rational method to reflect the distribution of unobservable inputs used to develop Level 3 fair value measurements. ASU 2018-13 is effective for us on January 1, 2020, with early adoption permitted. The Company has not yet determined the potential effects of ASU 2018-13 on its consolidated financial statements and disclosures.
In August 2018, the FASB issued ASU No. 2018-15, Intangibles—Goodwill and Other—Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract. The ASU aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. This new standard also requires customers to expense the capitalized implementation costs of a hosting arrangement that is a service contract over the term of the hosting arrangement. This ASU is effective for years beginning after December 15, 2020, with early adoption permitted. The Company has not yet determined the potential effects of this ASU on its consolidated financial statements.
In December 2019, the FASB issued Accounting Standard Update No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (ASU 2019-12), which eliminates certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also clarifies and simplifies other aspects of the accounting for income taxes. This guidance is effective for fiscal years beginning after December 31, 2021, and interim periods within fiscal years beginning after December 15, 2022. Early adoption is permitted. The Company is currently evaluating but has not yet determined the potential impact of the new standard on its consolidated financial statements.
In July 2017, the FASB issued ASU 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480) and Derivatives and Hedging (Topic 815): I. Accounting for Certain Financial Instruments with Down Round Features; II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception (“ASU 2017-11”). Part I of this update addresses the complexity of accounting for certain financial instruments with down round features. Down round features are features of certain equity- linked instruments (or embedded features) that result in the strike price being reduced on the basis of the pricing of future equity offerings. Current accounting guidance creates cost and complexity for entities that issue financial instruments (such as warrants and convertible instruments) with down round features that require fair value measurement of the entire instrument or conversion option. Part II of this update addresses the difficulty of navigating Topic 480, Distinguishing Liabilities from Equity, because of the existence of extensive pending content in the FASB Accounting Standards Codification. This pending content is the result of the indefinite deferral of accounting requirements about mandatorily redeemable financial instruments of certain nonpublic entities and certain mandatorily redeemable noncontrolling interests. The amendments in Part II of this update do not have an accounting effect. The amendments in Part I of this update are effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. Early adoption is permitted for all entities, including adoption in an interim period. The Company is currently assessing the potential impact of adopting ASU 2017-11 on its consolidated financial statements and disclosures.
F-24
In January 2017, the FASB adopted ASU 2017-04, Intangibles-Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment (“ASU 2017-04”) which simplifies how an entity is required to test goodwill for impairment by eliminating Step 2 from the goodwill impairment test. Step 2 measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of that goodwill. The implied fair value for a reporting unit is determined in the same manner as the amount of goodwill recognized in a business acquisition of the reporting unit. Under the amendments in ASU 2017-04, an entity shall recognize an impairment charge for the amount by which the carrying amount of a reporting unit exceeds its fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. The updated guidance requires adoption on a prospective basis. ASU 2017-04 is currently effective for the Company beginning January 1, 2022. Early adoption is permitted. The adoption of this standard update is not expected to have a material impact on our consolidated financial statements; however, any goodwill impairment losses recognized subsequent to adoption will be measured following the updated standard.
2. Acquisitions
Glen Research Corporation
In December 2017, the Company expanded its Nucleic Acid Production business by acquiring 100% of the outstanding equity of Glen Research via a stock purchase agreement (“Glen Research Purchase Agreement”). Pursuant to the Glen Research Purchase Agreement, all outstanding equity of Glen Research was acquired for total purchase consideration of $22.5 million.
Pursuant to the Glen Research Purchase Agreement, additional payments to the former owners of Glen Research were dependent on Glen Research meeting or exceeding defined revenue targets in each of 2018 and 2019 (together referred to as “the Glen Earn-Out”) and provided for a total maximum Glen Earn-Out payment of $4.0 million. As of December 31, 2018, the portion of the Glen Earn-Out relating to the 2018 defined revenue targets had been earned and therefore, was included in the current portion of contingent consideration in the related accompanying consolidated balance sheet. In addition, the fair value of the portion relating to the 2019 defined revenue targets, classified as long-term contingent consideration, was based on revised probabilities of this remaining contingent consideration being earned based on Glen Research revenue projections, expected payout term, and risk adjusted discount rates which were Level 3 inputs. The key quantitative assumption used in the determination of fair value as of December 31, 2018 included a discount rate of approximately 11%. During the year ended December 31, 2019, upon the achievement of 2019 defined revenue targets, the liability was revalued to reflect the maximum payout of $4.0 million under the provisions of the Glen Research Purchase Agreement. The change in fair value during 2018 and 2019, approximating $0.9 million and $0.3 million, respectively, has been reflected in the consolidated statement of operations within change in fair value contingent consideration. Upon achievement of the maximum $4.0 million payout, the outstanding liability is no longer considered a Level 3 liability under the fair value hierarch. During 2019, the first $2.0 million was paid to Glen Research, and the remaining $2.0 million, which is expected to be paid in 2020, has been reclassified to accrued expenses in our consolidated balance sheet (Note 4).
As of December 31, 2018, $2.1 million remained in the Indemnification Escrow account to secure the indemnification and certain other obligations of Glen Research, pursuant to the terms of the Purchase Agreement. The entire amount was released to the former owners in June 2019. Because these amounts held in escrow were not controlled by the Company, they were not included in the accompanying consolidated balance sheet at December 31, 2018.
TriLink BioTechnologies, LLC
In September 2016, and in connection with the establishment of the Nucleic Acid Production business, the Company acquired 100% of the outstanding equity of TriLink BioTechnologies via a securities purchase agreement (“TriLink Purchase Agreement”) which was partially financed with $38.6 million in third-party lender financing.
F-25
In May 2018, the intellectual property escrow of approximately $1.0 million was released to the sellers and the indemnification escrow of approximately $0.9 million was released to the Company. Pursuant to the TriLink Purchase Agreement, additional payments to the former owners of TriLink BioTechnologies were dependent on TriLink BioTechnologies meeting or exceeding defined revenue targets in each of 2017 and 2018 (the “Earn-Out”). The TriLink Purchase Agreement provided for a total maximum Earn-Out payment of $20.0 million. The portion of the Earn-Out relating to these executives has been recognized as compensation expense in the post-acquisition period, as earned. The remaining portion of the Earn-Out was recorded as contingent consideration and the fair value therein was included as part of the purchase consideration.
The Earn-Out liability of $5.8 million was paid in full during 2018, satisfying all related obligations.
The TriLink Purchase Agreement also provided for additional payments to the former owners of TriLink BioTechnologies of up to $10.0 million (the “customer holdback”). During the year ended December 31, 2017, the maximum customer holdback amount of $10.2 million was earned, of which $9.7 million was paid and approximately $0.5 million held back for certain seller related liabilities for pre-acquisition employee benefits the Company agreed to pay. The balance of $0.5 million and $0.3 million remained outstanding as of December 31, 2018 and 2019, respectively, and has been included in accrued expenses on the consolidated balance sheet.
3. Goodwill and Intangible Assets
The Company’s goodwill of $224.3 million as of December 31, 2018 and 2019, respectively, represents the excess of purchase consideration over the fair value of assets acquired and liabilities assumed. Given the lack of any triggering events being identified indicating that the fair value of the goodwill may be impaired, the Company completed its qualitative goodwill impairment analysis for the nucleic acid production and biologics safety testing reporting units during the fourth quarters of 2019 and 2018 and concluded it was not more-likely-than-not that the fair value of goodwill exceeded its carrying value and no further testing was required. Having identified triggering events for the protein detection reporting unit, the Company performed a quantitative analysis and also concluding that it was not more-likely-than-not that the fair value of goodwill exceeded its carrying value and no further testing was required. No asset impairment charges were recognized during either of the years ended December 31, 2018 or 2019.
The following is a rollforward of the Company’s goodwill by segment (amount in thousands):
| Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Total | |||||||||||||
| Balance, January 1, 2018 |
$ | 33,000 | $ | 119,928 | $ | 71,509 | $ | 224,437 | ||||||||
| Completion of purchase accounting for 2017 acquisition |
(162 | ) | — | — | (162 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance, December 31, 2018 |
$ | 32,838 | $ | 119,928 | $ | 71,509 | $ | 224,275 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
During the year ended December 31, 2019, there was no change in the recorded segment goodwill balances.
Intangible assets are being amortized on a straight-line basis, which reflects the expected pattern in which the economic benefits of the intangible assets are being obtained, over an estimated useful life ranging from 5 to 15 years.
F-26
The components of finite-lived intangible assets and accumulated amortization are as follows:
| As of December 31, 2018 | ||||||||||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Estimated Useful Life |
Weighted Average Remaining Amortization Period |
||||||||||||||||
| (in thousands) | (in years) | (in years) | ||||||||||||||||||
| Trade Names |
$ | 11,490 | $ | 2,801 | $ | 8,689 | 5-15 | 8.3 | ||||||||||||
| Patents and Developed Technology |
169,313 | 27,472 | 141,841 | 5-14 | 11.5 | |||||||||||||||
| Customer Relationships |
83,290 | 15,693 | 67,597 | 10-14 | 10.8 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 264,093 | $ | 45,966 | $ | 218,127 | 11.1 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| As of December 31, 2019 | ||||||||||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Estimated Useful Life |
Weighted Average Remaining Amortization Period |
||||||||||||||||
| (in thousands) | (in years) | (in years) | ||||||||||||||||||
| Trade Names |
$ | 11,490 | $ | 4,093 | $ | 7,397 | 5-15 | 7.3 | ||||||||||||
| Patents and Developed Technology |
169,313 | 40,134 | 129,179 | 5-14 | 10.5 | |||||||||||||||
| Customer Relationships |
83,290 | 22,013 | 61,277 | 10-14 | 9.8 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 264,093 | $ | 66,240 | $ | 197,853 | 10.1 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
The Company recognized $12.1 million and $12.2 million of amortization expense from intangible assets directly linked with revenue generating activities within cost of revenue in the consolidated statement of operations for the years ended December 31, 2018 and 2019, respectively. Amortization expense for intangible assets that are not directly related to sales generating activities of $8.0 million was recorded to selling, general and administrative expenses for each of the years ended December 31, 2018 and 2019.
As of December 31, 2019, the estimated future amortization expense for finite-lived intangible assets is as follows (in thousands):
| 2020 |
$ | 20,191 | ||
| 2021 |
20,079 | |||
| 2022 |
19,428 | |||
| 2023 |
19,230 | |||
| 2024 |
19,230 | |||
| Thereafter |
99,695 | |||
|
|
|
|||
| Total estimated amortization expense |
$ | 197,853 | ||
|
|
|
4. Fair Value Measurements
The table below presents the Company’s liabilities measured at fair value on a recurring basis aggregated by the level in the fair value hierarchy for the year ended December 31, 2018 (in thousands):
| Fair Value Measurements | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: |
||||||||||||||||
| Contingent consideration liabilities |
$ | — | $ | — | $ | 3,678 | $ | 3,678 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | — | $ | — | $ | 3,678 | $ | 3,678 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-27
There were no material assets or liabilities measured at fair value on a recurring basis as of December 31, 2019.
The Company assesses the fair value of contingent consideration to be settled in cash related to acquisitions using probability weighted models for the various contractual earn-outs. These are Level 3 measurements. Significant unobservable inputs used in the estimated fair values of these contingent consideration liabilities include probabilities of achieving customer related performance targets, specified sales milestones, changes in projected revenue and risk adjusted discount rates.
The following table provides a reconciliation of liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) for the years ended December 31, 2018 and 2019 (in thousands):
| Contingent Consideration |
||||
| Balance at January 1, 2018 |
$ | 8,558 | ||
| Change in fair value |
939 | |||
| Settlement |
(5,819 | ) | ||
|
|
|
|||
| Balance at December 31, 2018 |
$ | 3,678 | ||
| Change in fair value |
322 | |||
| Settlement |
(2,000 | ) | ||
| Transfer out of Level 3 fair value hierarchy |
(2,000 | ) | ||
|
|
|
|||
| Balance at December 31, 2019 |
$ | — | ||
|
|
|
|||
During the year ended December 31, 2019, upon the achievement of the maximum liability threshold of $4.0 million, as defined in the 2017 asset purchase agreement, the total contingent purchase consideration was no longer based on significant unobservable inputs. During 2019 the first $2.0 million was paid to Glen Research, and the remaining $2.0 million, which is expected to be paid in the second half of 2020, has been reclassified out of the Level 3 fair value hierarchy to other current liabilities in our consolidated balance sheet as of December 31, 2019.
5. Balance Sheet Components
Inventory
Inventory consists of the following at December 31 (in thousands):
| 2018 | 2019 | |||||||
| Raw materials |
$ | 4,766 | $ | 5,037 | ||||
| Work in process |
4,495 | 6,083 | ||||||
| Finished goods |
5,047 | 3,082 | ||||||
|
|
|
|
|
|||||
| $ | 14,308 | $ | 14,202 | |||||
|
|
|
|
|
|||||
F-28
Property and equipment
Property and equipment consists of the following at December 31 (in thousands):
| 2018 | 2019 | |||||||
| Land |
$ | 8,514 | $ | 8,516 | ||||
| Buildings |
7,604 | 7,685 | ||||||
| Buildings capitalized under lease finance obligations |
2,165 | 61,202 | ||||||
| Leasehold improvements |
2,092 | 2,990 | ||||||
| Furniture, fixtures, and equipment |
8,663 | 12,887 | ||||||
| Software |
1,035 | 1,742 | ||||||
|
|
|
|
|
|||||
| Total |
30,073 | 95,022 | ||||||
| Less accumulated depreciation |
(4,289 | ) | (8,099 | ) | ||||
|
|
|
|
|
|||||
| Total |
25,784 | 86,923 | ||||||
| Construction in-progress |
1,419 | 7,388 | ||||||
| Building and related construction in-progress improvements capitalized under a lease financing transaction (Note 6) |
15,375 | — | ||||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 42,578 | $ | 94,311 | ||||
|
|
|
|
|
|||||
Depreciation expense totaled approximately $2.2 million and $3.8 million for the years ended December 31, 2018 and 2019, respectively.
Accrued expenses
Accrued expenses consisted of the following at December 31 (in thousands):
| 2018 | 2019 | |||||||
| Employee related |
$ | 6,560 | $ | 7,660 | ||||
| Professional services |
337 | 3,199 | ||||||
| Sales and use tax liability |
2,074 | 3,030 | ||||||
| Accrued interest |
826 | 475 | ||||||
| Consideration payable |
— | 2,000 | ||||||
| Other |
1,350 | 2,151 | ||||||
|
|
|
|
|
|||||
| Total accrued expenses |
$ | 11,147 | $ | 18,515 | ||||
|
|
|
|
|
|||||
6. Commitments and Contingencies
Lease Commitments
The Company leases five facilities, including office, laboratory and manufacturing space under long-term non-cancelable operating leases. The leased facilities have initial terms of two to twelve years, and two leases have multiple five-year renewal terms and the other leases having three-year and five-year renewal terms. The Company also has capital leases for office equipment within initial terms of two to three years expiring in 2023.
Rent expense for each of the years ended December 31, 2018 and 2019 was approximately $2.0 and $2.5 million, respectively.
Prior to the Company’s October 2016 acquisition of MLSC, the Southport, North Carolina facility (the “Southport Facility”) leased by a subsidiary of MLSC failed to qualify for sale and leaseback accounting. As a result, MLSC recognized during construction, and retained upon the completion of construction, the value of the Southport Facility and obligation on its balance sheet as a financing obligation. Pursuant to the business combination fair value guidance, upon acquisition of MLSC, the Company recorded the fair value of the building asset, which was estimated to be $2.2 million, and the related financing obligation of $2.2 million.
F-29
In 2017, the Company amended its initial lease with the current related party landlord (Note 11) to include the lease of additional space as well as an adjustment of the base rent for the existing space. The Company continues to recognize payments under the amended lease agreement as a reduction of the facility financing obligation using the effective interest method and the ground rent as operating lease expense as notes in the schedule below. As a result of the amendment, the Company anticipates the repayment of the financing obligation by September 2024. The fair value of the leased property established at acquisition continues to be depreciated over the building’s estimated useful life of thirty-five years. At the conclusion of the lease term, the Company will de-recognize both the then carrying values of the asset and financing obligation with any differences between the book value of the building asset and remaining facility financing obligation being recognized in operations at that time. For its existing arrangement for the Southport Facility, these differences are expected to be immaterial. Payments on these lease obligations for the years ended December 31, 2018 and 2019 were approximately $0.3 million. For the years ended December 31, 2018 and 2019, rent expense associated with the ground lease for the Southport Facility was not significant.
In July 2018, the Company entered into a lease for a new manufacturing facility (the “San Diego Facility Lease”). The lease included tenant improvement provisions for construction prior to occupancy. Construction on this new manufacturing facility was in progress as of December 31, 2018. As of December 31, 2018, the Company evaluated the extent of its financial and operational involvement in the tenant improvements of the new facility related to the San Diego Facility Lease to determine whether it was considered the owner of the construction project under GAAP. The Company concluded that it was deemed to be the owner of the facility for accounting purposes (even though it did not meet the definition for legal purposes) during the construction period. As of December 31, 2018, the Company recognized the fair value of the building, additional construction costs incurred to date, and related construction payable liability of approximately $15.4 million in the accompanying consolidated balance sheet. The fair value of the leased building of $11.8 million included in fixed assets was estimated using a market approach that utilized comparable observable sales for similar assets (Level 2 inputs). As of December 31, 2018, the Company had also recognized building improvements totaling $3.6 million for additions to the leased building incurred by the Company during the construction period.
During 2018, the Company recognized $0.4 million of rent expense in the consolidated statement of operations associated with the ground lease for this new manufacturing facility. During 2018, the Company was not required to recognize interest expense on the cost of the construction payable due to its short-term nature. The allocation of the San Diego Facility Lease payment to ground lease rent expense and principal and interest expense on the lease financing obligation was estimated using income and market approaches that utilized comparable observable sales for similar assets, land capitalization rates and an estimate of the Company’s incremental borrowing rate (Level 2 and Level 3 inputs).
In 2019, upon completion of the construction, the Company evaluated the lease and concluded that the completed construction project failed to qualify for sale and leaseback accounting primarily due to the $8.0 million of non-recourse financing the Company provided to the lessor by reimbursing them for the $8.0 million of construction costs. The Company has accounted for the lease as a financing lease transaction. The leased building and related improvements remain on the Company’s balance sheet as of December 31, 2019 and rental payments associated with the San Diego Facility Lease have been allocated to operating lease expense for the ground underlying the leased building and principal and interest payments on the Lease Facility Financing Obligation. The Company recorded the fair value of the building asset and improvements, which was estimated to be $59.0 million and the related lease facility financing obligation of $51.2 million. The difference between the gross asset value and the lease facility financing obligation represents the approximate $8.0 million of building improvement costs reimbursed by the Company.
The Company recognizes payments under the lease agreement as a reduction of the lease facility financing obligation using the effective interest method and the ground rent as operating lease expense as reflected in the schedule below. The allocation of the San Diego Facility Lease payment to ground lease rent expense and principal and interest expense on the lease facility financing obligation was estimated using income and market
F-30
approaches that utilized comparable observable sales for similar assets, land capitalization rates, and an estimate of the Company’s incremental borrowing rate (Level 2 and Level 3 inputs). The fair value of the leased property, less its expected residual value, is depreciated over the term of the lease. At the conclusion of the lease term, the Company will de-recognize both the then carrying values of this asset and lease facility financing obligation with any differences between the book value of the asset and remaining lease facility financing obligation being recognized in operations at that time. For the Company’s arrangement for the San Diego Facility Lease these differences are expected to be immaterial. Payments on the San Diego Facility Lease obligation for the year ended December 31, 2019 were approximately $0.9 million. For the years ended December 31, 2018 and 2019, the Company recognized rent expense associated with the ground lease for the San Diego Facility Lease of approximately $0.4 million and $0.8 million, respectively, in the consolidated statement of operations.
As of December 31, 2019, minimum annual payments under the Company’s non-cancelable lease agreements, capital lease agreements, and lease financing obligations are as follows (in thousands):
| Capital Leases |
Lease Facility Financing Obligations |
Operating Leases |
||||||||||
| 2020 |
$ | 74 | $ | 2,116 | $ | 1,130 | ||||||
| 2021 |
52 | 3,853 | 1,091 | |||||||||
| 2022 |
21 | 4,101 | 1,153 | |||||||||
| 2023 |
1 | 4,401 | 1,203 | |||||||||
| 2024 |
— | 4,479 | 1,234 | |||||||||
| 2025 and beyond |
— | 25,155 | 5,579 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total minimum payments |
148 | 44,105 | $ | 11,390 | ||||||||
|
|
|
|||||||||||
| Less: amount representing interest |
(42 | ) | (30,566 | ) | ||||||||
|
|
|
|
|
|||||||||
| Present value of future minimum lease payments |
106 | 13,539 | ||||||||||
| Residual value of lease facility financing obligation |
— | 39,558 | ||||||||||
| Less: short-term capital lease and lease facility financing obligations |
(50 | ) | (178 | ) | ||||||||
|
|
|
|
|
|||||||||
| Long-term capital lease and lease facility financing obligations |
$ | 56 | $ | 52,919 | ||||||||
|
|
|
|
|
|||||||||
Operating leases in the table above includes future minimum lease payments for the ground lease for the Southport Facility and San Diego Facility Lease.
Legal Proceedings
The Company is involved in various legal proceedings arising in the normal course of business. The Company accrues for a loss contingency when it determines that it is probable, after consultation with counsel, that a liability has been incurred and the amount of such loss can be reasonably estimated. The Company believes that the results of any such contingencies, either individually or in the aggregate, will not have a material adverse effect on the Company’s financial position, results of operations or cash flows.
Indemnification Agreements
In the ordinary course of business, we may provide indemnification of varying scope and terms to vendors, lessors, customers and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from intellectual property infringement claims made by third parties. These indemnities include indemnities to our directors and officers to the maximum extent permitted under applicable state laws. The maximum potential amount of future payments that we could be required to make under these indemnification agreements is, in many cases, unlimited. We have not incurred any material costs as a result of such indemnifications and are not currently aware of any indemnification claims.
F-31
7. Long-Term Debt
As of December 31, 2017, the Company had term loan and revolving obligations outstanding under an amended and restated credit agreement as well as senior subordinated notes outstanding under an amended and restated note purchase agreement. Additionally, as of December 31, 2017, a subsidiary of MLSC also had a separate term loan and revolving loan obligations outstanding under an amended note purchase agreement. These four outstanding agreements (collective referred to as the “2017 Combined Debt Agreements”) remained outstanding until August 2, 2018.
In August 2018, Maravai Intermediate Holdings, LLC (“Intermediate”), a wholly-owned subsidiary of the Company, along with its subsidiaries (the “Borrowers”), entered into a First Lien Credit Agreement (the “First Lien Agreement”) and a Second Lien Credit Agreement (the “Second Lien Agreement”) (collectively referred to as the “First and Second Lien Credit Agreements”) with lending institutions for term-loan borrowings (“First Lien Term Loan”) totaling $250.0 million and for loans (“Second Lien Loan”) totaling $100.0 million, (collectively referred to as the “First and Second Lien Loans”) to refinance the 2017 Combined Debt Agreements (the “refinancing”), including repayment of all outstanding senior secured credit facilities and subordinated notes outstanding, and to allow for a $52.0 million distribution to the member of the Company. The First Lien Credit Agreement also provides for a revolving credit facility (the “Revolving Credit Facility”) of $50.0 million for letters of credit and loans to be used for working capital and other general corporate financing purposes. Borrowings under the First and Second Lien Loans are unconditionally guaranteed by the Company, immediate parent of Intermediate, along with the existing and future domestic subsidiaries of the Company (subject to certain exceptions) as specified in the respective guaranty agreements, and are secured by a lien and security interest in substantially all of the assets of existing and future domestic subsidiaries of the Company that are loan parties.
The refinancing of the previous debt agreements was evaluated for modification and extinguishment accounting. Certain lenders participated in the Company’s debt structure prior to the refinancing as well as in the Company’s debt structure after the refinancing. For these lenders, to the extent that it was concluded that the present value of cash flows under the terms of the First and Second Lien Loans differed by less than 10% from the present value of the remaining cash flows of the previous debt agreements, the refinancing was accounted for as a modification of the related outstanding debt balances and for the remaining of these lenders, whose cash flows changed by greater than 10% on a present value basis, the refinancing was accounted for as an extinguishment of the related debt. Certain prior lenders did not participate in the refinancing and the repayment of their related outstanding debt balances has been accounted for as an extinguishment of debt. Proceeds of borrowings from new lenders was accounted for as a new debt financing. As part of the refinancing, the Company incurred various costs including a $4.0 million original issue discount, $6.6 million in third-party debt issuance costs, and a $1.6 million prepayment penalty related to the senior subordinated notes. Of this $12.2 million of costs, the entire $4.0 million original issue discount, $4.8 million of third-party debt issuance costs, and $0.8 million of the prepayment penalty has been capitalized in the accompanying balance sheet within long-term debt, which is subject to amortization over the term of the refinanced debt as an adjustment to interest expense using the effective interest method. Of the remaining $2.6 million of costs incurred, $0.9 million was associated with the extinguished debt and included in the loss on extinguishment of debt in the accompanying consolidated statement of operations for the year ended December 31, 2018 and $1.7 million was related to the modified debt and immediately expensed to interest expense in the accompanying consolidated statement of operations for the year ended December 31, 2018. In addition, immediately prior to the refinancing, the Company had $7.1 million of unamortized issuance costs and discounts associated with the previous debt agreements, of which $4.7 million related to the extinguished debt and has been included in the loss on extinguishment of debt in the accompanying consolidated statement of operations for the year ended December 31, 2018 and $2.4 million related to the modified debt, which remains capitalized in the accompanying consolidated balance sheet as a component of long-term debt subject to amortization over the term of the refinanced debt. The First Lien Term Loan is repayable in quarterly payments of $0.6 million beginning December 31, 2018 through June 30, 2025, with all remaining outstanding principal due in August 2025. The First Lien Term Loan includes prepayment provisions that allow the Company, at our option, to repay all or a portion of the principal amount at
F-32
any time. During the first twelve months after August 2018, prepayments are subject to a 1.00% prepayment premium. The Revolving Credit Facility allows the Borrowers to repay and borrow from time to time until August 2023, at which time all amounts borrowed must be repaid. Subject to certain exceptions and limitations, the Company is required to repay borrowings under the First Lien Term Loan and Revolving Credit Facility with the proceeds of certain occurrences, such as the incurrence of debt, certain equity contributions, and certain asset sales or dispositions.
Borrowings under the First Lien Credit Agreement bear interest (a) in the cash of the First Lien Term Loans, at the Borrowers’ option either at (i) the Base Rate plus the applicable margin of 3.25% per annum based on the Company’s net leverage ratio or (ii) The Adjusted Eurocurrency Rate plus the margin of 4.25% per annum and (b) in the case of the Revolving Credit Facility, at the Borrowers’ option, either at (i) the Base Rate plus the applicable margin of 3.25% per annum with a stepdown to 3.00% based on the Company’s first lien net leverage ratio or (ii) the Adjusted Eurocurrency Rate plus the margin of 4.25% per annum with a stepdown to 4.00% based on the Company’s first lien net leverage ratio. The Base Rate is defined as the greatest of (a) the rate last quoted by The Wall Street Journal as the “Prime Rate” in the United States, (ii) the Federal Funds Rate plus 0.50% per annum, and (iii) the Adjusted Eurocurrency Rate for a one month interest period plus 1.00% per annum, (iv) solely with respect to the initial term loan. The Adjusted Eurocurrency Rate is defined as the greater of (a) with respect to the initial term loans the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate (as such term is defined in the First Lien Credit Agreement), and (ii) 1.00% and (b) with respect to the Revolving Credit Facility, the Eurocurrency Rate for such interest period (which if negative will be deemed to be 0%) multiplied by the Statutory Reserve Rate. The “Eurocurrency Rate” is defined as the London Inter-bank Offered Rate (LIBOR) as displayed by Reuters (which if negative will be deemed to be 0%).
At December 31, 2018 and 2019, the interest rate on the First Lien Term Loan was 6.8125% and 6.0625%, respectively.
Accrued interest under the First Lien Credit Agreement is payable (a) quarterly in arrears with respect to Base Rate loans, (b) at the end of each interest rate period (or at each three-month interval in the case of loans with interest periods greater than three months) with respect to Eurocurrency Rate loans, (c) on the date of any repayment or prepayment, and (d) at maturity (whether by acceleration or otherwise). An annual commitment fee is applied to the daily unutilized amount under the Revolving Credit Facility at 0.50% per annum, with one stepdown to 0.375% per annum based on the Company’s first lien net leverage ratio.
The Second Lien Credit Agreement includes voluntary prepayment provisions that allow the Borrowers, at their option, to repay all or a portion of the principal amount of the loans between August 2, 2019 and August 2, 2020, subject to a prepayment premium of 1.00% of the principal amount repaid.
If a change of control (as defined in the Amended and Restated Note Purchase Agreement) occurs, the Company must repay the outstanding principal amount plus a premium (calculated the same as redemption premium) and accrued and unpaid interest.
Borrowings under the Second Lien Credit Agreement bear interest, at the Company’s option either at (a) the Base Rate plus the applicable margin of 7.0% per annum or (b) Adjusted Eurocurrency Rate plus the margin of 8.00% per annum. The “Base Rate” is defined as the greatest of (i) the rate last quoted by The Wall Street Journal as the “Prime Rate” in the United States, (ii) the Federal Funds Rate plus 0.50%, and (iii) Adjusted Eurocurrency Rate for a one month interest period plus 1% per annum, (iv) soley with respective to the initial term loans, 2.00% per annum and (v) for any loans that are not initial term loans, 1.00% per annum. The “Adjusted Eurocurrency Rate” is defined as (a) with respect to the initial term loans the greater of (i) the Eurocurrency Rate for such interest period multiplied by the Statutory Reserve Rate, and (ii) 1.00% and (b) with respect to the Revolving Credit Facility, the Eurocurrency Rate for such interest period (which if negative will be deemed to be 0%) multiplied by the Statutory Reserve Rate. The “Eurocurrency Rate” is defined as the London Inter-bank Offered Rate (LIBOR) as displayed by Reuters (which if negative will be deemed to be 0%).
F-33
At December 31, 2018 and 2019, the interest rate on the Second Lien Loan was 10.4% and 9.7%, respectively.
Accrued interest on Borrowings under the Second Lien Credit Agreement is payable (a) quarterly in arrears with respect to Base Rate loans, (b) at the end of each interest rate period (or at each three-month interval in the case of loans with interest periods greater than three months) with respect to Eurocurrency Rate Loans, (c) the date of any repayment or prepayment, and (iv) at maturity (whether by acceleration or otherwise).
Debt Covenants
The First Lien Credit Agreement requires that, as of the end of each fiscal quarter the net leverage ratio of Intermediate shall not be greater than 7.50 to 1.00.
The First and Second Lien Credit Agreements also contain negative and affirmative covenants in addition to the financial covenant in the First Lien Credit Agreement, including covenants that restrict the ability of the Company and its subsidiaries ability to, among other things, incur or prepay existing indebtedness, pay dividends or distributions, dispose of assets, engage in mergers and consolidations, make acquisitions or other investments, and make changes in the nature of the business. The First and Second Lien Credit Agreements contain certain events of default, including, without limitation, nonpayment of principal, interest or other obligations, violation of the covenants, insolvency, court ordered judgments, and certain changes of control. The First and Second Lien Credit Agreements also require the Company to provide audited consolidated financial statements to the lenders no later than 120 days after year-end.
The First Lien Credit Agreement also requires mandatory prepayments to be calculated in 2019 upon certain excess cash flow as defined in the terms of the agreement to be paid beginning in 2020. As of December 31, 2019, no mandatory prepayment was required.
The Borrowers were in compliance with all of their debt covenants under the First and Second Lien Credit Agreements as of December 31, 2018 and 2019 and there were no events of default for the years ended December 31, 2019.
The Company’s total debt at December 31 consisted of (in thousands):
| 2018 | 2019 | |||||||
| First Lien Term Loan |
$ | 249,375 | $ | 246,875 | ||||
| Second Lien Loan |
100,000 | 100,000 | ||||||
| Unamortized debt issuance costs |
(11,325 | ) | (9,592 | ) | ||||
|
|
|
|
|
|||||
| Total long-term debt |
338,050 | 337,283 | ||||||
| Less: current portion |
(2,500 | ) | (2,500 | ) | ||||
|
|
|
|
|
|||||
| Total long-term debt, less current portion |
$ | 335,550 | $ | 334,783 | ||||
|
|
|
|
|
|||||
As of December 31, 2019, the aggregate future principal maturities of the Company’s debt obligations for each of the next five years, based on contractual due dates, are as follows (in thousands):
| 2020 |
$ | 2,500 | ||
| 2021 |
2,500 | |||
| 2022 |
2,500 | |||
| 2023 |
2,500 | |||
| 2024 |
2,500 | |||
| Thereafter |
334,375 | |||
|
|
|
|||
| Total debt |
$ | 346,875 | ||
|
|
|
F-34
Interest Rate Cap Agreements
As of December 31, 2018 and 2019, the Company has entered into four interest rate cap agreements with a financial institution to manage its variable interest rate risk on a portion of its credit borrowings under the First and Second Lien Credit Agreements. As of December 31, 2018 and 2019, the fair value of the Company’s interest rate cap agreements were insignificant and were included in other non-current assets in the accompanying consolidated balance sheets. The change in fair value during the years ended December 31, 2018 and 2019 was also insignificant and was recognized as other income (expense) in the accompanying consolidated statement of operations.
8. Member’s Equity
Pursuant to the Company’s limited liability company agreement, the Company has established a single class of common units with MLSH 1 as its sole member. The Company is authorized to issue up to 253,916,941 common units. All authorized 253,916,941 common units have been issued and are outstanding. MLSH 1 as the member, is not obligated to make capital contributions to the Company. The Company’s profits and losses shall be allocated to MLSH 1 as determined by the Board of Directors. MLSH 1, as the member of the Company, is not liable, solely by reason of being a member, for the debts, obligations, or liabilities of the Company whether arising in contract or tort under judgment, decree, or other of a court; or otherwise. The Company will dissolve only upon the written consent of MLSH 1. The Company’s common units have no conversion rights and no explicit redemption rights. No capital contributions were received by the Company from MLSH 1 in 2018 or 2019
Pursuant to the Company’s limited liability company agreement, distributions to MLSH 1 shall be made at the discretion of the Company’s the Board of Directors.
The Company’s Board of Directors approved and distributions were made to MLSH 1 in the amount of $52.1 million during the year-ended December 31, 2018. No distributions were made to MLSH 1 during the year ended December 31, 2019
MLSC Incentive Units
The Company’s majority-owned subsidiary, MLSC, is authorized and at the discretion of the MLSC Board, under its Limited Liability Company Agreement (“MLSC LLC Agreement”), as amended in October 2016, to issue common units that can be designated as Incentive Units. The Incentive Units may be subject to either a combination of service, market or performance vesting conditions. Vested Incentive Units are treated as common units for purposes of distributions.
During the year ended December 31, 2017, MLSC awarded 1,820,000 incentive units (“MLSC Incentive Units”) to several employees of its subsidiary, of which 364,000 included performance condition vesting. Awards which vest based solely on a service condition provide for cliff-vesting over five years. The MLSC Incentive Units that include performance conditions tied to the achievement of certain cash distribution multiples provide for full vesting upon meeting the performance condition. No compensation cost has been recorded for the MLSC Incentive Units with performance conditions as achieving the cash distribution multiples performance condition associated with these awards was not considered probable.
All vested MLSC Incentive Units are subject to repurchase for fair value at MLSC’s option upon a voluntary or involuntary separation event that is not deemed to be for cause and only after seven months have passed since the separation event. During 2018 and 2019, the Company repurchased 12,000 and 160,000, respectively, MLSC Incentive Units for $0.2 million and an insignificant amount, respectively.
F-35
MLSC Incentive Unit activity during the periods indicated is as follows:
| Number of Unvested MLSC Incentive Units |
Weighted Average Grant Date Fair Value Per Unit |
|||||||
| Balance as of January 1, 2018 |
2,539,800 | $ | 0.88 | |||||
| MLSC Units forfeited |
(340,000 | ) | 0.90 | |||||
| MLSC Units vested |
(729,200 | ) | 0.87 | |||||
|
|
|
|||||||
| Balance as of December 31, 2018 |
1,470,600 | $ | 0.87 | |||||
| MLSC Units vested |
(649,200 | ) | 0.87 | |||||
|
|
|
|||||||
| Balance as of December 31, 2019 |
821,400 | $ | 0.88 | |||||
|
|
|
|||||||
No MLSC Incentive Units were granted in 2018 or 2019.
Unit-based compensation expense for the years ended December 31, 2018 and 2019 was approximately $0.6 million and $0.4 million, respectively.
As of December 31, 2018 and 2019, there were 2,905,000 and 2,745,000 and MLSC Incentive Units outstanding, respectively, of which 1,470,600 and 821,400 remained unvested, respectively. The total fair value of the MLSC Incentive Units that vested in 2018 and 2019 was approximately $0.6 million in each of the periods. At December 31, 2018, and 2019, 12.1 million additional MLSC units were available for grant.
MLSH 1 Incentive Units
The Company has entered into agreements with certain executives and board members whereby those employees and board members were granted incentive units in MLSH 1, the Company’s parent and sole member (“MLSH 1 Incentive Units”). All MLSH 1 Incentive Unit awards are subject to a market condition which is subject to the achievement of a certain investment return threshold that increases on a compounding basis annually and a service condition subject to their continued employment. Certain MLSH 1 Incentive Unit awards also contain performance conditions tied to the consummation of a business acquisition. Other MLSH 1 Incentive Unit awards contain a performance condition tied to the achievement of certain cash distribution multiples. The fair value of MLSH 1 Incentive Unit awards is measured at the grant date and is recognized as expense over the requisite service period for the awards.
During the years ended December 31, 2018 and 2019, all MLSH 1 Incentive Unit awards with performance conditions were subject solely to the achievement of defined cash distribution multiples. No compensation cost has been recorded for the MLSH 1 Incentive Unit awards with performance conditions as achieving the cash distribution multiples performance condition associated with these awards was still not considered probable.
Total compensation cost recognized by the Company during each of the years ended December 31, 2018 and 2019, for all MLSH 1 Incentive Unit awards, was approximately $1.5 million and $1.3 million and, respectively.
During the year ended December 31, 2018, a total of 23,000 MLSH 1 Incentive Unit awards were granted of which 3,000 included performance condition vesting. During the year ended December 31, 2019, a total of 169,500 MLSH 1 Incentive Unit awards were granted of which 34,500 included performance condition vesting. The MLSH 1 Incentive Unit awards that include market and service conditions provide for cliff-vesting generally over four or five years. The MLSH 1 Incentive Unit awards that include market and performance conditions provide for full vesting upon meeting the performance condition. No compensation cost has been recorded for the MLSH 1 Incentive Unit awards with performance conditions as achieving performance conditions associated with these awards was still not considered probable.
F-36
All vested MLSH 1 Incentive Unit awards are subject to repurchase for fair value at the Company’s option upon a voluntary or involuntary separation event that is not deemed to be for cause. No MLSH 1 Incentive Unit awards were repurchased in 2018. During 2019, MLSH 1 repurchased 1,000 MLSH 1 Incentive Unit awards for an insignificant amount.
MLSH 1 Incentive Unit award activity during the periods indicated is as follows:
| Number of Unvested MLSH 1 Incentive Unit awards |
Weighted Average Grant Date Fair Value |
|||||||
| Balance as of January 1, 2018 |
975,500 | $ | 8.37 | |||||
| MLSH 1 Incentive Unit awards granted |
23,000 | 10.53 | ||||||
| MLSH 1 Incentive Unit awards forfeited |
(35,000 | ) | 6.69 | |||||
| MLSH 1 Incentive Unit awards vested |
(168,500 | ) | 6.33 | |||||
|
|
|
|||||||
| Balance as of December 31, 2018 |
795,000 | $ | 8.91 | |||||
| MLSH 1 Incentive Unit awards granted |
169,500 | 18.60 | ||||||
| MLSH 1 Incentive Unit awards forfeited |
(6,500 | ) | 11.68 | |||||
| MLSH 1 Incentive Unit awards vested |
(170,900 | ) | 6.38 | |||||
|
|
|
|||||||
| Balance as of December 31, 2019 |
787,100 | $ | 11.53 | |||||
|
|
|
|||||||
As of December 31, 2018 and 2019, there were 1,215,000 and 1,377,500 MLSH 1 Incentive Unit awards outstanding of which and 795,000 and 787,100 had not yet vested, respectively. The fair value of the units underlying the MLSH 1 Incentive Unit awards was estimating using the aggregate implied equity value of MLSH 1 using a weighting of discounted cash flow analysis, comparable public company analysis and comparable transactions analysis. An Option-Pricing Method (“OPM”) was then used to allocate the total equity value of MLSH 1 to the different classes of equity according to their rights and preferences. To apply the OPM, volatility was estimated based on the historical volatility of similar public companies’ stock price over a preceding period commensurate with the expected term of the MLSH 1 Incentive Unit awards. The Company estimated the expected term of the MLSH 1 Incentive Unit awards was estimated considering the timing and probabilities of a liquidity event. The risk-free interest rate for the expected term of the MLSH 1 Incentive Unit awards was based on the U.S. Treasury yield curve in effect at the time of grant. Compensation expense related to the MLSH 1 Incentive Unit awards during the years ended December 31, 2018 and 2019 were $0.4 million and an insignificant amount, respectively.
The following table sets forth the compensation expense related to both the MLSC Incentive Units and MLSH 1 Incentive Unit awards included in the accompanying consolidated statements of operations for the year ended December 31 (in thousands):
| 2018 | 2019 | |||||||
| Cost of revenue |
$ | 38 | $ | 22 | ||||
| Research and development |
297 | 211 | ||||||
| Selling, general and administrative |
1,786 | 1,446 | ||||||
|
|
|
|
|
|||||
| $ | 2,121 | $ | 1,679 | |||||
|
|
|
|
|
|||||
At December 31, 2018 and 2019, there was $2.8 million and $3.7 million of total unrecognized compensation cost related to unvested MLSH 1 Incentive Units and MLSC Incentive Unit awards not subject to a performance condition that is expected to be recognized over a weighted average period of 2.5 and 1.9 years, respectively.
F-37
As of December 31, 2018 and 2019, there was $3.6 million and $4.1 million, respectively, of unrecognized compensation cost associated with the total of all MLSH 1 Incentive Units and MLSC Incentive Unit awards subject to a performance condition.
9. Income Taxes
The Company and a number of its subsidiaries are treated as flow-through entities for federal income tax purposes. The income or loss generated by these entities are not taxed at the LLC level. As required by U.S. tax law, income or loss generated by these LLCs flows through to MLSH 1, the Company’s sole member. As such, the Company’s income tax provision consists solely of the activities of its taxable subsidiaries which are taxed as corporations for federal income tax purposes.
The components of loss from operations for the Company’s taxable subsidiaries before provision for income taxes for the years ended December 31 are as follows (in thousands):
| 2018 | 2019 | |||||||
| U.S. |
$ | (2,471 | ) | $ | (2,484 | ) | ||
| International |
(206 | ) | (272 | ) | ||||
|
|
|
|
|
|||||
| $ | (2,677 | ) | $ | (2,756 | ) | |||
|
|
|
|
|
|||||
Income tax expense (benefit) consisted of the following for the years ended December 31 (in thousands):
| 2018 | 2019 | |||||||
| Current tax expense |
||||||||
| Federal |
$ | 87 | $ | 505 | ||||
| State |
6 | 2 | ||||||
| International |
— | — | ||||||
|
|
|
|
|
|||||
| 93 | 507 | |||||||
|
|
|
|
|
|||||
| Deferred tax expense (benefit) |
||||||||
| Federal |
847 | (1,224 | ) | |||||
| State |
(554 | ) | 65 | |||||
| International |
31 | — | ||||||
|
|
|
|
|
|||||
| 324 | (1,159 | ) | ||||||
|
|
|
|
|
|||||
| Total income tax expense (benefit) |
$ | 417 | $ | (652 | ) | |||
|
|
|
|
|
|||||
A reconciliation between the Company’s effective tax rate and the applicable U.S. federal statutory income tax rate as of December 31 (in thousands) is summarized as follows:
| 2018 | 2019 | |||||||
| Federal statutory rate |
21.0 | % | 21.0 | % | ||||
| State and local taxes, net of federal benefits |
20.5 | % | (2.4 | %) | ||||
| Deferred tax revaluation |
(52.1 | %) | (0.1 | %) | ||||
| One-time transition tax |
(4.3 | %) | — | |||||
| Unit-based compensation |
(2.4 | %) | (1.7 | %) | ||||
| Research and development credit |
2.9 | % | 1.9 | % | ||||
| Intercompany sale of inventory |
1.0 | % | 1.2 | % | ||||
| Uncertain tax positions |
(0.6 | %) | 5.4 | % | ||||
| Other |
(1.6 | %) | (1.6 | %) | ||||
|
|
|
|
|
|||||
| Effective tax rate |
(15.6 | %) | 23.7 | % | ||||
|
|
|
|
|
|||||
F-38
Deferred tax assets and liabilities as of December 31 (in thousands) are summarized as follows:
| 2018 | 2019 | |||||||
| Deferred tax assets: |
||||||||
| Interest limitation |
$ | 1,331 | $ | 2,333 | ||||
| NOL and credit carryforwards |
982 | 455 | ||||||
| Accruals |
458 | 454 | ||||||
| Inventories |
362 | 19 | ||||||
| Other |
109 | — | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
3,242 | 3,261 | ||||||
| Valuation allowance |
(1,565 | ) | (1,561 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax assets, net of valuation allowance |
$ | 1,677 | $ | 1,700 | ||||
|
|
|
|
|
|||||
| Deferred tax liabilities: |
||||||||
| Intangibles |
$ | (14,127 | ) | $ | (13,007 | ) | ||
| Property and equipment |
(3,089 | ) | (3,064 | ) | ||||
| Transaction costs |
(303 | ) | (326 | ) | ||||
| Other |
(14 | ) | — | |||||
|
|
|
|
|
|||||
| Total deferred tax liabilities |
(17,533 | ) | (16,397 | ) | ||||
|
|
|
|
|
|||||
| Total net deferred tax liabilities |
$ | (15,856 | ) | $ | (14,697 | ) | ||
|
|
|
|
|
|||||
The Company’s valuation allowance primarily relates to Internal Revenue Code (“IRC”) 163(j) interest expense and insignificant foreign NOL carryforwards. As of December 31, 2018 the Company recorded a full valuation allowance against its IRC 163(j) interest expense carryforward based on negative evidence associated with the Company’s projection of income and financing plans. As of December 31, 2019, the Company only recorded a partial valuation allowance against IRC 163(j) due to expected future income generated by the sale of land, building and related building improvements in 2020 (Note 14). The net change in valuation allowance was insignificant for the year ended December 31, 2019 due to increases in the IRC 163(j) and foreign NOL carryforwards offset by the release in valuation allowance on the 163(j) carryforward due to expected future taxable income.
Based on its previous indefinite reinvestment assertion, the Company has not historically provided deferred taxes on earnings in certain non-U.S. subsidiaries because such earnings were intended to be indefinitely reinvested in its international operations. With the introduction of a modified territorial tax system in the 2017 Tax Act, the Company has reviewed its previously stated intent and no longer indefinitely reinvests the undistributed earnings of its foreign operations. For the years ending December 31, 2018 and 2019, the amount of undistributed foreign earnings was $1.4 million and $1.2 million, respectively. The Company believes any unrecorded liabilities related with these earnings are not material.
As of December 31, 2018, the Company had U.S. Federal NOL carryforwards of $2.7 million. As of December 31, 2019, the Company had no U.S. Federal NOL carryforwards. As of December 31, 2018 and 2019, the Company had state NOL carryforwards of $2.4 million and $1.4 million, respectively, available to reduce future taxable income. State NOL carryforwards to future periods begin to expire in 2034. As of December 31, 2018 and 2019, the Company had no U.S. Federal Research and Development tax credit carryforwards. As of December 31, 2018 and 2019, the Company had California Research and Development Tax Credit carryforwards of $0.1 million and $0.1 million, respectively, the California Research and Development Tax Credits are not subject to expiration.
On June 29, 2020, Assembly Bill 85 (“A.B. 85”) was signed into California law. A.B. 85 provides for a three-year suspension of the use of net operating losses for medium and large businesses and a three-year cap on the use of business incentive tax credits to offset no more than $5.0 million of tax per year. A.B. 85 suspends the use of net operating losses for taxable years 2020, 2021 and 2022 for certain taxpayers with taxable income of $1.0 million or more. The carryover period for any net operating losses that are suspended under this provision will be extended.
F-39
A.B. 85 also requires that business incentive tax credits including carryovers may not reduce the applicable tax by more than $5.0 million for taxable years 2020, 2021 and 2022. The Company is currently assessing the impact of adopting this standard on its consolidated financial statements but does not expect the impact to be material.
The Company had foreign NOL carryforwards of $1.0 million and $1.3 million for the years ending December 31, 2018 and 2019, respectively. Substantially all of the foreign NOLs do not have an expiration date.
In response to the COVID-19 pandemic, the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”) and the Families First Coronavirus Response Act (“FFCR Act”) were signed into law in March 2020. The CARES Act lifts certain deduction limitations originally imposed by the 2017 Tax Act. Corporate taxpayers may carryback NOLs originating during 2018 through 2020 for up to five years, which was not previously allowed under the 2017 Tax Act. The CARES Act also eliminates the 80% of taxable income limitations by allowing corporate entities to fully utilize NOL carryforwards to offset taxable income in 2018, 2019 or 2020. Taxpayers may generally deduct interest up to the sum of 50% of adjusted taxable income plus business interest income (30% limit under the 2017 Tax Act) for tax years beginning January 1, 2019 and 2020. The CARES Act allows taxpayers with alternative minimum tax credits to claim a refund in 2020 for the entire amount of the credits instead of recovering the credits through refunds over a period of years, as originally enacted by the 2017 Tax Act. The CARES Act and FFCR Act also included provisions related to refundable payroll tax credits, deferment of employer side social security payments.
In addition, the CARES Act raises the corporate charitable deduction limit to 25% of taxable income and makes qualified improvement property generally eligible for 15-year cost-recovery and 100% bonus depreciation. The CARES Act will be applicable to the Company in 2020 and is expected to affect the Company’s ability to utilize certain tax credit carryforwards related to the deduction of interest that were limited under the 2017 Tax Act. The expected impact on the 2020 consolidated financial statements approximates $1.0 million. The Company is also evaluating other effects the CARES Act and FFCR Act will have on its consolidated financial statements.
As of December 31, 2018 and 2019, the Company had $0.1 million and $0.2 million, respectively, of unrecognized tax benefits, all of which would affect its effective tax rate if recognized. We do not expect any significant increases or decreases to our unrecognized tax benefits in the next twelve months. The Company recognizes interest as a component of income tax expense.
The aggregate changes in the balance of the Company’s unrecognized tax benefits, as of December 31, were as follows (in thousands):
| 2018 | 2019 | |||||||
| Balance, beginning of year |
$ | 106 | $ | 136 | ||||
| Gross increases based on tax positions related to current year |
34 | 5 | ||||||
| Gross increase based on tax positions related to prior years |
— | 105 | ||||||
| Gross decreases based on tax positions related to prior years |
(4 | ) | (38 | ) | ||||
|
|
|
|
|
|||||
| Balance, end of year |
$ | 136 | $ | 208 | ||||
|
|
|
|
|
|||||
The Company files income tax returns in the U.S. federal jurisdiction, California, Canada, and the United Kingdom and is not under audit by any taxing authority in any of these jurisdictions. In the normal course of business the Company is subject to examination by taxing authorities throughout the world. With a few exceptions, the Company is no longer subject to U.S. federal, state, and local, or non-U.S. income tax examinations for years before 2015 except for utilization of NOL carryforwards.
10. Net Loss per Common Unit Attributable to the Member of Maravai
Net loss per common unit attributable to our member for the years ended December 31, 2018 and 2019 is based on the weighted average number of common units outstanding during the period. The members’ equity of MLSC is
F-40
comprised of Class A and Class B preferred units, MLSC Incentive Units and common units, each with participation rights. The MLSC preferred units are entitled to cumulative dividends of 8.0% compounded annually, up to an additional 4.0%, also compounded annually, to the extent of remaining unallocated earnings. The preferred unitholders of MLSC are required, however, to share a portion of the additional 4.0% in dividends with the holders of MLSC Incentive Units based on a formula defined in the MLSC LLC Agreement. The Company determined that vested MLSC Incentive Units and MLSC Class A and B Preferred Units are participating securities under the two-class method at the MLSC subsidiary level, however, they do not have a contractual obligation to share in losses, and therefore no undistributed losses have been allocated to them. MLSH 1 Incentive Units are granted by the parent of the Company, and as a result, do not represent potential common units of the Company.
Diluted net loss per common unit attributable to our member is computed by adjusting the net loss and the weighted-average number of common units outstanding to give effect to potentially dilutive securities. The Company has issued potentially dilutive instruments in the form of MLSC Incentive Units granted to employees and officers of MLSC. The Company did not include these MLSC Incentive Units in its calculation of diluted loss per unit during the years ended December 31, 2018 and 2019, because to include them would be anti-dilutive due to the Company’s net loss during such periods.
The following table sets forth the computation of basic and diluted net loss per common unit attributable to our member for the years ended December 31 (in thousands, except units and per unit amounts):
| 2018 | 2019 | |||||||
| Net loss per common unit—basic and diluted: |
||||||||
| Net Loss |
$ | (16,915 | ) | $ | (5,201 | ) | ||
| Less: preferred unit dividends attributable to noncontrolling interests |
(5,259 | ) | (5,680 | ) | ||||
| Add: loss attributable to common noncontrolling interests |
4,446 | 2,400 | ||||||
|
|
|
|
|
|||||
| Net loss attributable to the Company common unitholder |
$ | (17,728 | ) | $ | (8,481 | ) | ||
|
|
|
|
|
|||||
| Weighted average common units outstanding |
253,916,941 | 253,916,941 | ||||||
| Net loss per common unit—basic and diluted: |
$ | (0.07 | ) | $ | (0.03 | ) | ||
The following common unit equivalents have been excluded from the calculations of diluted net loss per common unit for the years ended December 31 because their inclusion would be antidilutive:
| 2018 | 2019 | |||||||
| Time-based incentive units |
2,896,000 | 2,896,000 | ||||||
| Performance-based incentive units |
249,000 | 249,000 | ||||||
|
|
|
|
|
|||||
| 3,145,000 | 3,145,000 | |||||||
|
|
|
|
|
|||||
11. Segments
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. When determining the reportable segments, the Company aggregated operating segments based on their similar economic and operating characteristics. Segment results are presented in the same manner as we present our operations internally to make operating decisions and assess performance. The accounting policies for the segments are the same as those described in Significant Accounting Policies (Note 1). The Company’s financial performance is reported in three segments. A description of each segment follows:
| • | Nucleic Acid Production: focuses on the manufacturing and sale of highly modified nucleic acids products to support the needs of customers’ research, therapeutic and vaccine programs. This segment also provides research products for labeling and detecting proteins in cells and tissue samples. |
F-41
| • | Biologics Safety Testing: focuses on manufacturing and selling biologics safety and impurity tests and assay development services that are utilized by our customers in their biologic drug manufacturing spectrum. |
| • | Protein Detection: focuses on manufacturing and selling labeling and visual detection reagents to scientific research customers for their tissue-based protein detection and characterization needs. |
The Company has determined that adjusted earnings before interest, tax, depreciation, and amortization (“Adjusted EBITDA”) is the profit or loss measure that the CODM uses to make resource allocation decisions and evaluate segment performance. Adjusted EBITDA assists management in comparing the segment performance on a consistent basis for purposes of business decision-making by removing the impact of certain items that management believes do not directly reflect the core operations and, therefore, are not included in measuring segment performance. The Company defines Adjusted EBITDA as net income before interest, taxes, depreciation and amortization, certain non-cash items, and other adjustments that we do not consider in our evaluation of ongoing operating performance from period to period. Corporate costs are managed on a standalone basis and not allocated to segments.
Following is financial information relating to the operating segments (in thousands):
| For the year ended December 31, 2018 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Total | |||||||||||||||
| Revenue |
$ | 60,057 | $ | 38,492 | $ | 25,284 | $ | — | $ | 123,833 | ||||||||||
| Adjusted EBITDA |
$ | 16,751 | $ | 31,199 | $ | 13,846 | $ | (8,796 | ) | $ | 53,000 | |||||||||
| For the year ended December 31, 2019 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Total | |||||||||||||||
| Revenue |
$ | 72,602 | $ | 44,416 | $ | 26,122 | $ | — | $ | 143,140 | ||||||||||
| Adjusted EBITDA |
$ | 22,229 | $ | 36,371 | $ | 14,603 | $ | (11,189 | ) | $ | 62,014 | |||||||||
There was no inter-segment activity for any of the periods presented and all of the revenue for each segment is from external customers.
The Company does not allocate assets to its reportable segments as they are not included in the review performed by the CODM for purposes of assessing segment performance and allocating resources. Excluding approximately $0.3 million associated with a building in the United Kingdom, all of the Company’s long-lived assets are located within the United States.
F-42
A reconciliation of Adjusted EBITDA to net loss, the most directly comparable GAAP measure, is set forth below (in thousands):
| Year Ended December 31, |
||||||||
| 2018 | 2019 | |||||||
| Net loss |
$ | (16,915 | ) | $ | (5,201 | ) | ||
| Add: |
||||||||
| Amortization |
20,122 | 20,274 | ||||||
| Depreciation |
2,225 | 3,810 | ||||||
| Interest expense |
27,399 | 29,959 | ||||||
| Income tax expense (benefit) |
417 | (652 | ) | |||||
|
|
|
|
|
|||||
| EBITDA |
33,248 | 48,190 | ||||||
| Acquisition contingent consideration |
939 | 322 | ||||||
| Loss on extinguishment of debt |
5,622 | — | ||||||
| Acquisition integration costs |
7,529 | 6,170 | ||||||
| Amortization of purchase accounting inventory step-up |
2,967 | 1,856 | ||||||
| Unit-based compensation |
2,121 | 1,679 | ||||||
| GTCR management fees |
574 | 523 | ||||||
| Merger and acquisition related expenses |
— | 3,274 | ||||||
|
|
|
|
|
|||||
| Adjusted EBITDA |
$ | 53,000 | $ | 62,014 | ||||
|
|
|
|
|
|||||
12. Employee Benefit Plans
The Company sponsors a 401(k) plan (the “Maravai LifeSciences 4019(k) Plan”) that stipulates that eligible employees can elect to contribute to the 401(k) Plan, subject to certain limitations, on a pretax basis. The Company provides for a match of up to 50% of employee contributions up to the first 6.0% of salary. The Company match vest over a four-year term. In February 2019, a retirement saving plan of one of our subsidiaries was closed and all funds in the plan were rolled over into the Maravai LifeSciences 401(k) Plan.
The Company also maintains a non-qualified Long-Term Incentive Plan (“LTIP”) for legacy employees of one of their subsidies which is not subject to the Employee Retirement Income Security Act of 1974.
Total contributions by the Company to these plans were approximately and $1.0 million and $1.2 million for the years ended December 31, 2018 and 2019, respectively.
13. Related Party Transactions
MLSH 1 has an advisory services agreement with GTCR, LLC, MLSH 1’s majority owner. Under this agreement GTCR provides the Company financial and management consulting services in the areas of corporate strategy, budgeting for future corporate investments, acquisition and divestiture strategies and debt and equity financings. The advisory services agreement provides that the Company pay placement fees to GTCR of 1.0% of the gross amount of any debt or equity financings. During the years ended December 31, 2018 and 2019, no placement fees were incurred. The advisory services agreement provides that the Company pay a $0.1 million quarterly management fee to GTCR commencing on the date of the first acquisition. For each of the years ended December 31, 2018 and 2019, the Company incurred approximately $0.5 million in management fees to GTCR which were paid in full as of December 31, 2019 and 2018, respectively.
The Company also reimburses GTCR for out-of-pocket expenses incurred while providing the above professional services. During the years ended December 31, 2018 and 2019, the Company incurred out-of-pocket expenses to GTCR of $0.1 million and $2.4 million, respectively. Of these balances, the amounts included in accrued expense at December 31, 2019 approximated $2.4 million. The amounts included in accounts payable and accrued expenses were insignificant as of December 31, 2018 and 2019.
F-43
The noncontrolling interests in MLSC represent equity interest that was retained by the shareholders of the MLSC entity prior to its acquisition by the Company. The President of Cygnus Technologies and his affiliated entity are the owners of the noncontrolling interests.
The Company leases a facility (Note 6), which is owned by an entity controlled by a close relative of the President of one of its subsidiaries. The close relative was also previously an employee of the Company who terminated their employment during the year ended December 31, 2018. The President of this subsidiary also personally financed a loan to this entity which was used to acquire the property leased by the Company. For the years ending December 31, 2018 and 2019, the Company paid $0.2 million in lease payments for the leased facility.
14. Subsequent Events
The Company has evaluated subsequent events through October 29, 2020 (except for the impact of the unit split as discussed in the fifth paragraph of Note 1, as to which the date is November 11, 2020) the date on which the consolidated financial statements were issued.
In January 2020, the Company completed the sale of land, building and related building improvements specific to its facility in Burlingame, California for approximately $25.2 million, net of tax. Simultaneously, with the close of the transaction, the Company leased the property for a two-and-a-half-year period. The future lease obligations under the lease, which were excluded from the future lease obligation table above in Note 6, approximate $3.1 million.
In March 2020, the Company acquired MockV Solutions, Inc. (“MockV”), a private entity, for total gross cash consideration equal to $3.0 million minus debt, transaction expenses, and half of the amount required to maintain the patents between signing and closing, plus cash held by MockV at the time of the acquisition. The acquisition also provides for the possibility of future contingent consideration up to $9.0 million, based on the entity’s achievement of long-term sales targets at various points of measurement, as defined by the purchase agreement.
In March 2020, the Company drew down approximately $15.0 million on its existing Revolving Credit Facility to provide financing for the acquisition of MockV and other operating uses.
In March 2020, the World Health Organization declared the global novel coronavirus disease 2019 (“COVID-19”), outbreak a pandemic. While certain impacts of COVID-19 have been favorable to sale of the Company’s products and services, Company cannot at this time predict the specific extent, duration, or full impact that the COVID-19 outbreak will have on its financial condition and operations. The impact of the COVID-19 coronavirus outbreak on the financial performance of the Company may depend on future developments, including the duration and spread of the outbreak and related governmental advisories and restrictions. In addition, the Company could see some limitations on employee resources that would otherwise be focused on our operation, including but not limited to sickness of employees or their families, the desire of employees to avoid contact with large groups of people, and increased reliance on working from home.
In September 2020, the Company amended its San Diego Facility lease agreement to provide for additional manufacturing and office space. The amended lease agreement provides for tenant improvements for construction prior to occupancy, rent concessions, and escalating rent payments over the life of the lease which now expires in May 2023. The total future minimum lease payments under the amended lease agreement are $55.6 million, with the option to renew subject to certain conditions.
F-44
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except unit amounts)
| December 31, 2019 |
September 30, 2020 |
Pro forma September 30, 2020 |
||||||||||
| Assets | (Unaudited) | (Unaudited) | ||||||||||
| Current Assets |
||||||||||||
| Cash |
$ | 24,700 | $ | 124,882 | $ | 36,314 | ||||||
| Accounts receivable, net |
18,030 | 73,301 | 73,301 | |||||||||
| Inventory |
14,202 | 26,586 | 26,586 | |||||||||
| Prepaid expenses and other current assets |
3,620 | 9,352 | 9,352 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
60,552 | 234,121 | 145,553 | |||||||||
| Property and equipment, net |
94,311 | 96,089 | 96,089 | |||||||||
| Goodwill |
224,275 | 224,275 | 224,275 | |||||||||
| Intangible assets, net |
197,853 | 182,820 | 182,820 | |||||||||
| Other assets |
805 | 807 | 807 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
$ | 577,796 | $ | 738,112 | $ | 649,544 | ||||||
|
|
|
|
|
|
|
|||||||
| Liabilities and members’ equity (deficit) |
||||||||||||
| Current liabilities |
||||||||||||
| Accounts payable |
$ | 7,478 | $ | 7,796 | $ | 7,796 | ||||||
| Accrued expenses |
18,515 | 33,683 | 33,683 | |||||||||
| Repurchase liability for incentive units |
— | 9,140 | 9,140 | |||||||||
| Repurchase liability for noncontrolling interests |
— | 166,427 | 166,427 | |||||||||
| Deferred revenue |
841 | 60,674 | 60,674 | |||||||||
| Other current liabilities |
228 | 231 | 231 | |||||||||
| Current portion of long-term debt |
2,500 | 2,500 | 2,500 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
29,562 | 280,451 | 280,451 | |||||||||
| Long-term debt, less current portion |
334,783 | 349,204 | 349,204 | |||||||||
| Deferred tax liabilities |
14,697 | 13,422 | 13,422 | |||||||||
| Lease facility financing obligation, less current portion |
52,919 | 56,440 | 56,440 | |||||||||
| Other long-term liabilities |
1,208 | 2,601 | 2,601 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
433,169 | 702,118 | 702,118 | |||||||||
|
|
|
|
|
|
|
|||||||
| Commitments and contingencies (Note 6) |
||||||||||||
| Member’s equity (deficit) |
||||||||||||
| Contributed capital, 253,916,941 units authorized, issued and outstanding |
183,910 | 14,777 | — | |||||||||
| Retained earnings (accumulated deficit) |
(42,381 | ) | 21,381 | (52,410 | ) | |||||||
| Accumulated other comprehensive loss |
(133 | ) | (164 | ) | (164 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total member’s equity (deficit) attributable to Maravai Topco Holdings, LLC member |
141,396 | 35,994 | (52,574 | ) | ||||||||
| Noncontrolling interests |
3,231 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total member’s equity (deficit) |
144,627 | 35,994 | (52,574 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities and member’s equity (deficit) |
$ | 577,796 | $ | 738,112 | $ | 649,544 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements
F-45
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except unit and per unit amounts)
(Unaudited)
| Nine Months Ended September 30, | ||||||||
| 2019 | 2020 | |||||||
| Revenue |
$ | 107,180 | $ | 185,745 | ||||
| Operating Expenses |
||||||||
| Cost of revenue |
49,019 | 56,254 | ||||||
| Research and development |
2,648 | 7,212 | ||||||
| Selling, general and administrative |
32,570 | 52,624 | ||||||
| Change in estimated fair value of contingent consideration |
241 | — | ||||||
| Gain on sale and leaseback transaction |
— | (19,002 | ) | |||||
|
|
|
|
|
|||||
| Total operating expenses |
84,478 | 97,088 | ||||||
|
|
|
|
|
|||||
| Income from operations |
22,702 | 88,657 | ||||||
| Other income (expense) |
||||||||
| Interest expense |
(22,240 | ) | (21,934 | ) | ||||
| Other income |
95 | 132 | ||||||
|
|
|
|
|
|||||
| Income before income taxes |
557 | 66,855 | ||||||
| Income tax expense |
308 | 2,511 | ||||||
|
|
|
|
|
|||||
| Net income |
249 | 64,344 | ||||||
| Net (loss) income attributable to noncontrolling interests |
(672 | ) | 582 | |||||
|
|
|
|
|
|||||
| Net income attributable to the Maravai Topco Holdings, LLC member |
$ | 921 | $ | 63,762 | ||||
|
|
|
|
|
|||||
| Net Income per common unit attributable to Maravai Topco Holdings, LLC member—basic and diluted |
$ | (0.01 | ) | $ | 0.21 | |||
|
|
|
|
|
|||||
| Weighted average common units outstanding |
253,916,941 | 253,916,941 | ||||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements
F-46
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in thousands)
(Unaudited)
| Nine Months Ended September 30, |
||||||||
| 2019 | 2020 | |||||||
| Net income |
$ | 249 | $ | 64,344 | ||||
| Other comprehensive income (loss) |
||||||||
| Foreign currency translation adjustments |
(21 | ) | (31 | ) | ||||
|
|
|
|
|
|||||
| Comprehensive income |
228 | 64,313 | ||||||
| Comprehensive (loss) income attributable to noncontrolling interests |
(672 | ) | 582 | |||||
|
|
|
|
|
|||||
| Comprehensive income attributable to Maravai Topco Holdings, LLC member |
$ | 900 | $ | 63,731 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements
F-47
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF MEMBER’S EQUITY
(in thousands except unit amounts)
(Unaudited)
| For the Nine Months Ended |
Units | Amount | Contributed Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Noncontrolling Interests |
Total Member’s Equity |
|||||||||||||||||||||
| December 31, 2018 |
253,916,941 | $ | — | $ | 182,809 | $ | (38,237 | ) | $ | (167 | ) | $ | 3,611 | $ | 148,016 | |||||||||||||
| Cumulative effect of adoption of ASC 606 |
— | — | — | 326 | — | — | 326 | |||||||||||||||||||||
| Repurchase of incentive units |
— | — | (227 | ) | — | — | — | (227 | ) | |||||||||||||||||||
| Unit-based compensation |
— | — | 899 | — | — | 264 | 1,163 | |||||||||||||||||||||
| Net income |
— | — | — | 921 | — | (672 | ) | 249 | ||||||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | — | (21 | ) | — | (21 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| September 30, 2019 |
253,916,941 | $ | — | $ | 183,481 | $ | (36,990 | ) | $ | (188 | ) | $ | 3,203 | $ | 149,506 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| For the Nine Months Ended |
Units | Amount | Contributed Capital |
Retained Earnings (Accumulated Deficit) |
Accumulated Other Comprehensive Loss |
Noncontrolling Interests |
Total Member’s Equity |
|||||||||||||||||||||
| December 31, 2019 |
253,916,941 | $ | — | $ | 183,910 | $ | (42,381 | ) | $ | (133 | ) | $ | 3,231 | $ | 144,627 | |||||||||||||
| Repurchase of incentive units |
— | — | (9,140 | ) | — | — | — | (9,140 | ) | |||||||||||||||||||
| Distributions to member |
— | — | (312 | ) | — | — | — | (312 | ) | |||||||||||||||||||
| Unit-based compensation |
— | — | 1,450 | — | — | 1,483 | 2,933 | |||||||||||||||||||||
| Net income |
— | — | — | 63,762 | — | 582 | 64,344 | |||||||||||||||||||||
| Recognition of repurchase liability for noncontrolling interests |
— | — | (161,131 | ) | — | — | (5,296 | ) | (166,427 | ) | ||||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | — | (31 | ) | — | (31 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| September 30, 2020 |
253,916,941 | $ | — | $ | 14,777 | $ | 21,381 | $ | (164 | ) | $ | — | $ | 35,994 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements
F-48
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(Unaudited)
| Nine Months Ended September 30, |
||||||||
| 2019 | 2020 | |||||||
| Operating activities |
||||||||
| Net income |
$ | 249 | $ | 64,344 | ||||
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: |
||||||||
| Depreciation |
2,331 | 4,756 | ||||||
| Amortization of intangible assets |
15,118 | 15,156 | ||||||
| Provision for doubtful accounts |
35 | 225 | ||||||
| Amortization of deferred financing costs |
1,297 | 1,296 | ||||||
| Unit-based compensation |
1,163 | 2,933 | ||||||
| Deferred income taxes |
— | (1,275 | ) | |||||
| Change in estimated fair value of contingent consideration |
241 | — | ||||||
| Gain on sale and leaseback transaction |
— | (19,002 | ) | |||||
| Acquired in-process research and development costs |
— | 2,881 | ||||||
| Other |
540 | 1,116 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable |
(2,583 | ) | (55,420 | ) | ||||
| Inventory |
(951 | ) | (12,384 | ) | ||||
| Prepaid expenses and other current assets |
(3,962 | ) | (971 | ) | ||||
| Other assets |
13 | — | ||||||
| Accounts payable |
911 | 130 | ||||||
| Accrued expenses and other current liabilities |
(549 | ) | 8,838 | |||||
| Other long-term liabilities |
(3 | ) | 300 | |||||
| Deferred revenue |
1,020 | 59,833 | ||||||
|
|
|
|
|
|||||
| Net cash provided by operating activities |
14,870 | 72,756 | ||||||
|
|
|
|
|
|||||
| Investing activities |
||||||||
| Cash paid for asset acquisition, net of cash acquired |
— | (3,024 | ) | |||||
| Purchases of property and equipment |
(12,388 | ) | (16,559 | ) | ||||
| Proceeds from sale of building |
— | 34,500 | ||||||
|
|
|
|
|
|||||
| Net cash (used in) provided by investing activities |
(12,388 | ) | 14,917 | |||||
|
|
|
|
|
|||||
| Financing activities |
||||||||
| Distribution to members |
— | (312 | ) | |||||
| Proceeds from borrowings of long term debt |
— | 15,000 | ||||||
| Repurchase of vested incentive units |
(227 | ) | — | |||||
| Principal repayments of long term debt |
(1,875 | ) | (1,875 | ) | ||||
| Payment of contingent consideration |
(2,000 | ) | — | |||||
| Payment for deferred financing costs |
— | (250 | ) | |||||
| Payments made on facility financing lease obligation and capital lease |
(109 | ) | (23 | ) | ||||
|
|
|
|
|
|||||
| Net cash (used in) provided by investing activities |
(4,211 | ) | 12,540 | |||||
| Effects of exchange rate changes on cash |
(21 | ) | (31 | ) | ||||
|
|
|
|
|
|||||
| Net (decrease) increase in cash |
(1,750 | ) | 100,182 | |||||
| Cash, beginning of period |
21,866 | 24,700 | ||||||
|
|
|
|
|
|||||
| Cash, end of period |
$ | 20,116 | $ | 124,882 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow information |
||||||||
| Cash paid for interest |
$ | 20,770 | $ | 19,407 | ||||
|
|
|
|
|
|||||
| Cash paid for income taxes |
$ | 492 | $ | 3,506 | ||||
|
|
|
|
|
|||||
| Supplemental disclosures of non-cash investing and financing activities |
|
|||||||
| Property and equipment included in accounts payable and accrued expenses |
$ | 1,049 | $ | 5,163 | ||||
|
|
|
|
|
|||||
| Building and improvements capitalized under lease financing transaction |
$ | 51,201 | $ | — | ||||
|
|
|
|
|
|||||
| Deferred financing costs included in accounts payable and accrued expenses |
$ | — | $ | 2,574 | ||||
|
|
|
|
|
|||||
| Repurchase liability for incentive units |
$ | — | $ | 9,140 | ||||
|
|
|
|
|
|||||
| Repurchase liability for noncontrolling interests |
$ | — | $ | 166,427 | ||||
|
|
|
|
|
|||||
| Receivable from lessor funded financing |
$ | — | $ | 2,686 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements
F-49
MARAVAI TOPCO HOLDINGS, LLC AND SUBSIDIARIES
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Significant Accounting Policies
Organization and Description of Business
Maravai Topco Holdings, LLC (“Maravai,” “we,” “our” or the “Company”) is a holding company focused on building a transformative life sciences products company by acquiring businesses and accelerating their growth through capital infusions and industry expertise. The Company was organized as a Delaware limited liability company (“LLC”) on July 27, 2018, as a wholly-owned subsidiary of Maravai Life Sciences Holdings, LLC (“MLSH 1”).
The Company is headquartered in San Diego, California and has three principal businesses: Nucleic Acid Production, Biologics Safety Testing, and Protein Detection. Our Nucleic Acid Production business manufactures and sells products used in the fields of gene therapy, nucleoside chemistry, oligonucleotide therapy and diagnostics, including reagents used in the chemical synthesis, modification, labelling and purification of deoxyribonucleic acid (“DNA”) and ribonucleic acid (“RNA”). Our Biologics Safety Testing business sells highly specialized analytical products for use in biologic manufacturing process development, including custom product-specific development antibody and assay development services. Our Protein Detection business sells innovative labeling and detection reagents for researchers in immunohistochemistry.
In October 2016, the Company acquired a controlling interest in MLSC Holdings, LLC (“MLSC”), and the parent entity of one of our biologics safety testing operating companies, by purchasing approximately 70% of the equity interests of MLSC with the remaining 30% being recorded as noncontrolling interests in the consolidated financial statements of the Company. In September 2020, MLSH 1 entered into a Sale and Rollover Agreement with the President of Cygnus Technologies and other current unit holders of MLSC (collectively, the “Investors”) to repurchase the outstanding MLSC Incentive Units, Class B preferred units and MLSC common units from the Investors (see Note 8).
Basis of Presentation
The Company’s condensed consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). The accompanying condensed consolidated financial statements include the accounts of the Company and its wholly-owned and majority-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
The Company believes that its existing cash at September 30, 2020 will be sufficient to allow the Company to fund its current operating plan through at least a period of one year after the date the condensed consolidated financial statements are issued.
Unit Split
On November 11, 2020, the Company’s member approved an amendment to the Company’s limited liability company agreement to increase the authorized common units from 1,000 to 253,916,941 and effect a 253,916.941-for-1 split of its common units. All of the Company’s unit and per unit information included in the accompanying condensed consolidated financial statements has been adjusted to reflect the split.
Unaudited Interim Condensed Financial Statements
The accompanying condensed consolidated balance sheet as of September 30, 2020, the condensed consolidated statements of operations and cash flows for the nine months ended September 30, 2019 and 2020,
F-50
the condensed consolidated statement of member’s equity for the nine months ended September 30, 2019 and 2020, and the related interim condensed consolidated disclosures are unaudited. In management’s opinion, the unaudited condensed consolidated financial statements have been prepared on the same basis as the annual financial statements for the years ended December 31, 2019 and 2020, and include all adjustments necessary to state fairly the financial position as of September 30, 2020; the results of operations and cash flows for the nine months ended September 30, 2019 and 2020; and the condensed consolidated statement of member’s equity for the nine months ended September 30, 2020. The consolidated balance sheet as of December 31, 2019 included herein was derived from the audited financial statements as of that date. The results for the nine months ended September 30, 2019 and 2020 are not necessarily indicative of the operating results to be expected for the full fiscal year or any future period. Certain information and footnote disclosures normally included in annual financial statements prepared in accordance with GAAP have been condensed or omitted. Therefore, these interim condensed financial statements should be read in conjunction with the Company’s audited financial statements included elsewhere in this prospectus.
Unaudited Pro Forma Condensed Consolidated Balance Sheet Information
The Company paid $88.6 million of distributions to MLSH 1 in October 2020. The distribution was fully funded through the use of cash on hand as of September 30, 2020 and cash flows generated from a refinancing of the Company’s debt in October 2020 (see Note 14). For purposes of the unaudited pro forma condensed consolidated balance sheet, the payment of the distributions is reflected as a reduction to member’s equity of $73.8 million, contributed capital of $14.8 million and a reduction to cash of $88.6 million. The pro forma balances do not give effect to the proceeds from the refinancing but reflects the distributions as if such distributions were declared and paid on September 30, 2020.
Use of Estimates
The preparation of consolidated financial statements in accordance with GAAP requires the Company to make estimates and assumptions that affect the reported amounts in the consolidated financial statements and accompanying notes. These estimates form the basis for judgments the Company makes about the carrying values of assets and liabilities that are not readily apparent from other sources. The Company bases its estimates and judgments on historical experience and on various other assumptions that the Company believes are reasonable under the circumstances. These estimates are based on management’s knowledge about current events and expectations about actions the Company may undertake in the future. Significant estimates include, but are not limited to, revenue recognition, the net realizable value of inventory, expected future cash flows including growth rates, discount rates, terminal values and other assumptions and estimates used to evaluate the recoverability of long-lived assets, estimated fair values of intangible assets and goodwill, amortization methods and periods, valuation of intangible assets, the fair value of leased buildings and other assumptions associated with lease financing transactions, the estimated fair value of our long-term debt, unit-based compensation, the valuation of our and MLSH 1’s incentive units, allowance for doubtful accounts, and accounting for income taxes. Actual results could differ materially from those estimates.
Revenue
The Company generates revenue from the sale of products and services and the performance of services in the fields of nucleic acid production, biologics safety testing, and protein detection. Revenue is recognized when control of promised goods or services is transferred to a customer in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To determine revenue recognition for its arrangements with customers, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. The majority of the Company’s contracts include only one performance obligation. A performance obligation is a promise in a contract to transfer a distinct good or service
F-51
to the customer and is defined as the unit of account for revenue recognition. The Company also recognizes revenue from other contracts that may include a combination of products and services, the provision of solely services, or from license fee arrangements which may be associated with the delivery of product. Where there is a combination of products and services, the Company accounts for the promises as individual performance obligations if they are concluded to be distinct. Performance obligations are considered distinct if they are both capable of being distinct and distinct within the context of the contract. In determining whether performance obligations meet the criteria for being distinct, the Company considers a number of factors, such as the degree of interrelation and interdependence between obligations, and whether or not the good or service significantly modifies or transforms another good or service in the contract. As a practical expedient, we do not adjust the transaction price for the effects of a significant financing component if, at contract inception, the period between customer payment and the transfer of goods or services is expected to be one year or less. Contracts with customers are evaluated on a contract-by-contract basis as contracts may include multiple types of goods and services as described below.
Nucleic Acid Production
Nucleic acid production revenue is generated from the manufacture and sale of highly modified, complex nucleic acids products to support the needs of our customers’ research, therapeutic and vaccine programs. The primary offering of products include; CleanCap®, mRNA, and specialized oligonucleotides. Contracts typically consist of a single performance obligation. We also sell nucleic acid products for labeling and detecting proteins in cells and tissue samples research. The Company recognizes revenue from these products in the period in which the performance obligation is satisfied by transferring control to the customer. Revenue for nucleic acid catalog products is recognized at a single point in time, generally upon shipment to the customer. Revenue for contracts for certain custom nucleic acid products, with an enforceable right to payment and a reasonable margin for work performed to date, is recognized over time, based on a cost-to-cost input method over the manufacturing period.
Biologics Safety Testing
The Company’s biologics safety testing revenue is associated with the sale of bioprocess impurity detection kit products. We also enter into contracts that include custom antibody development, assay development and antibody affinity extraction services. These products and services enable the detection of impurities and contaminants that occur in the manufacturing of biologic drugs and other therapeutics. The Company recognizes revenue from the sale of bioprocess impurity detection kits in the period in which the performance obligation is satisfied by transferring control to the customer. Custom antibody development contracts consist of a single performance obligation, typically with an enforceable right to payment and a reasonable margin for work performed to date. Revenue is recognized over time based on a cost-to-cost input method over the contract term. Where an enforceable right to payment does not exist, revenue is recognized at a point in time when control is transferred to the customer. Assay development service contracts consist of a single performance obligation, revenue is recognized at a point in time when a successful antigen test and report is provided to the customer. Affinity extraction services, which generally occur over a short period of time, consist of a single performance obligation to perform the extraction service and provide a summary report to the customer. Revenue is recognized either over time or at a point in time depending on contractual payment terms with the customer. The Company also has certain licensing and royalty arrangements with an immaterial amount of revenue.
Protein Detection
The Company also manufactures and sells protein labeling and detection reagents to customers that are used for basic research and development. The contracts to sell these catalog products consist of a single performance obligation to deliver the reagent products. Revenue from these contracts is recognized at a point in time, generally upon shipment of the final product to the customer.
F-52
The Company recognizes royalty revenue related to certain out-licensing and royalty arrangements in the period the sales or usage occur using third-party evidence to estimate the amount to be recorded. To date this revenue has not been material to the consolidated financial statements.
The Company has elected the practical exemption to not disclose the unfulfilled performance obligations for contracts with an original length of one year or less. The Company has no material unfulfilled performance obligations for contracts with an original length greater than one year at December 31, 2019 and September 30, 2020.
The Company accepts returns only if the products do not meet customer specifications and historically, the Company’s volume of product returns has not been significant. Further, no warranties are provided for promised goods and services other than assurance type warranties.
Revenue for an individual contract is recognized at the related transaction price, which is the amount the Company expects to be entitled to in exchange for transferring the products and/or services. The transaction price for product sales is calculated at the contracted product selling price. The transaction price for a contract with multiple performance obligations is allocated to the separate performance obligations on a relative standalone selling price basis. Standalone selling prices for products are determined based on the prices charged to customers, which are directly observable. Standalone selling price of services are mostly based on time and materials. Generally, payments from customers are due when goods and services are transferred. As most contracts contain a single performance obligation, the transaction price is representative of the standalone selling price charged to customers. Revenue is recognized only to the extent that it is probable that a significant reversal of the cumulative amount recognized will not occur in future periods. Since the adoption of ASC 606, variable consideration has not been material.
Sales taxes
Sales taxes collected by the Company are not included in the transaction price as revenue they are ultimately remitted to a governmental authority.
Shipping and handling costs
The Company has elected to account for shipping and handling activities related to contracts with customers as costs to fulfill the promise to transfer the associated products. Accordingly, revenue for shipping and handling is recognized at the same time that the related product revenue is recognized.
Contract costs
The Company recognizes the incremental costs of obtaining contracts as an expense when incurred when the amortization period of the assets that otherwise would have been recognized is one year or less. These costs are included in sales and marketing and general and administrative expenses. The costs to fulfill the contracts are determined to be immaterial and are recognized as an expense when incurred.
Contract balances
Contract assets are generated when contractual billing schedules differ from revenue recognition timing and the Company records contract receivable when it has an unconditional right to consideration. As of December 31, 2019 and September 30, 2020, the contract assets, which are included in prepaid and other current assets, both totaled $0.8 million.
Contract liabilities are recorded when cash payments are received or due in advance of performance. Contract liabilities consist of customer deposits, which are included in accrued expenses, and deferred revenue, where the
F-53
Company has unsatisfied performance obligations. As of December 31, 2019 and September 30, 2020, the contract liabilities were $1.0 million and $61.9 million, respectively, with the contract liabilities at December 31, 2019 expected to be recognized into revenue in the year ended December 31, 2020 and the contract liabilities at September 30, 2020 expected to be recognized into revenue in the year ended December 31, 2021.
Disaggregation of Revenue
The following tables summarize the revenue by segment and region (in thousands):
| For the nine months ended September 30, 2019 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Total | ||||||||||||
| North America |
$ | 37,244 | $ | 13,991 | $ | 11,749 | $ | 62,984 | ||||||||
| Europe, the Middle East, and Africa |
12,573 | 8,980 | 4,924 | 26,477 | ||||||||||||
| Asia Pacific |
5,032 | 9,521 | 2,626 | 17,179 | ||||||||||||
| Latin and Central America |
18 | 327 | 195 | 540 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
$ | 54,867 | $ | 32,819 | $ | 19,494 | $ | 107,180 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| For the nine months ended September 30, 2020 |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Total | ||||||||||||
| North America |
$ | 82,523 | $ | 16,217 | $ | 9,577 | $ | 108,317 | ||||||||
| Europe, the Middle East, and Africa |
32,731 | 10,974 | 4,002 | 47,707 | ||||||||||||
| Asia Pacific |
13,296 | 13,356 | 2,730 | 29,382 | ||||||||||||
| Latin and Central America |
19 | 225 | 95 | 339 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
$ | 128,569 | $ | 40,772 | $ | 16,404 | $ | 185,745 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table provides a disaggregation of revenue for the nine months ended September 30, based on the pattern of revenue recognition (in thousands):
| 2019 | 2020 | |||||||
| Revenue recognized at a point in time |
$ | 98,500 | $ | 181,041 | ||||
| Revenue recognized over time |
8,680 | 4,704 | ||||||
|
|
|
|
|
|||||
| Total revenue |
$ | 107,180 | $ | 185,745 | ||||
|
|
|
|
|
|||||
Unit-Based Compensation
The Company’s parent, MLSH 1, grants unit-based awards to certain executives of the Company in the form of non-vested units. Our controlled subsidiary, MLSC, grants unit-based awards only to certain employees of its subsidiaries (collectively, the “Incentive Units”). All awards of Incentive Units are measured based on the fair value of the award on the date of grant. The Company recognizes compensation expense for MLSH 1 awards in its consolidated financial statements as MLSH 1 is considered to be the economic interest holder in the Company. Compensation expense for the Incentive Units is recognized over their requisite service period. Forfeitures are recognized when they occur. These Incentive Units are subject to service, market and performance conditions. For Incentive Units subject to performance conditions, the Company evaluates the probability of achieving each performance condition at each reporting date and recognizes expense over the requisite service period when it is deemed probable that a performance condition will be met using the accelerated attribution method over the requisite service period.
The grant date fair value of Incentive Unit awards has been determined by the Company’s Board of Directors with the assistance of management and an independent third-party valuation specialist. The grant date
F-54
fair value of Incentive Units was determined first by estimating an aggregate equity value using a weighting of discounted cash flows, comparable public companies, and comparable-transactions valuation methodologies. An Option-Pricing Method or Probability Expected Return Method, which utilize certain assumptions including, probability weighting of events, volatility, time to liquidation, a risk-free interest rate, and an assumption for a discount for lack of marketability, was then used to allocate the total equity value of the Company to the different classes of equity according to their rights and preferences. In determining the fair value of the Incentive Units, the methodologies used to estimate the enterprise value of the Company were performed using methodologies, approaches, and assumptions consistent with the American Institute of Certified Public Accountants Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation (“AICPA Accounting and Valuation Guide”).
Income Taxes
The Company’s wholly-owned U.S. subsidiary, Maravai Life Sciences, Inc. (“Maravai Inc.”) and certain wholly-owned subsidiaries of the Company, are taxpaying entities in the U.S., Canada, and the U.K. Accordingly, the Company provides current and deferred income taxes for these entities. The Company and its other subsidiaries are treated as pass-through entities for federal and state income tax purposes and are not subject to income tax as the LLC member is responsible for the tax consequences of its proportionate share of the pass-through income or loss. As such, the Company’s tax provision consists solely of the activities of Maravai Inc. and certain wholly-owned subsidiaries of the Company, which are taxed as a corporation for federal and state income tax purposes.
The Company’s taxable subsidiaries account for income taxes using the asset and liability method, under which deferred tax liabilities and assets are recognized for the expected future tax consequences of temporary differences between the consolidated financial statement carrying amounts and the tax basis of assets and liabilities and net operating loss (“NOL”) and tax credit carryforwards. Deferred tax assets and liabilities are classified as noncurrent on the consolidated balance sheet. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. The Company uses a recognition threshold and measurement attribute for the consolidated financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. A tax position is recognized when it is more likely than not that the tax position will be sustained upon examination, including the resolution of any related appeals or litigation. A tax position that meets the more likely-than-not recognition threshold is measured at the largest amount of benefit that is greater than a 50% likelihood of being realized upon ultimate settlement with a taxing authority. Interest and penalties related to unrecognized tax benefits are recognized in benefit from income taxes in the accompanying consolidated statements of operations and comprehensive loss. No such interest and penalties were recognized for any period presented.
Noncontrolling Interests
Noncontrolling interests represent the portion of profit or loss, net assets and comprehensive loss that is not allocable to the member of Maravai. Noncontrolling interests arise from the Company’s majority-owned subsidiary, MLSC, in which the Company holds a 70% ownership interest. MLSC is not liable, solely by reason of being a member, for the debts, obligations, or liabilities of the Company whether arising in contract or tort; under a judgment, decree, or order of a court; or otherwise. MLSC net income or loss is attributed to the noncontrolling interests using an attribution method, similar to the hypothetical liquidation at book value method, based on the distribution provisions of the MLSC Amended and Restated Limited Liability Company Agreement (“MLSC LLC Agreement”). In September 2020, the Company entered into a transaction to repurchase these remaining noncontrolling interests in MLSC (see Note 9).
Segment Information
The Company operates in three reportable segments. Operating segments are defined as components of an enterprise about which separate financial information is evaluated regularly by the chief operating decision
F-55
maker in deciding how to allocate resources and assessing performance. The Company’s chief operating decision maker (“CODM”), its Chief Executive Officer, allocates resources and assesses performance based upon discrete financial information at the segment level. Substantially all of our long-lived assets are located in the United States.
Net Income per Common Unit Attributable to the Member
Basic net income per common unit attributable to the member of Maravai is calculated by dividing the net income adjusted for the Company’s noncontrolling interests by the weighted-average number of common units outstanding during the period. The noncontrolling interests is calculated pursuant to the terms of the MLSC LLC Agreement, the Company’s majority-owned subsidiary, on a fully-distributed basis, taking into account the various classes of equity of MLSC, including the cumulative yields on MLSC’s preferred units. Diluted net income per common unit attributable to the member of Maravai is computed by using the weighted-average number of common units outstanding during the period and the potential dilutive common unit equivalents as determined under the two-class method. In periods in which the Company reports a net loss attributable to the member of Maravai, diluted net loss per common unit is the same as basic net loss per common unit attributable to the member of Maravai since dilutive common units are not assumed to have been issued if their effect is antidilutive.
For the nine months ended September 30, 2019 and 2020, the Company reported net income attributable to the member of Maravai.
Deferred Offering Costs
Deferred offering costs, which consist of direct incremental legal, consulting, banking and accounting fees primarily relating to the Company’s contemplated initial public offering (“IPO”), are capitalized and will be offset against proceeds upon the consummation of the offering within member’s equity. In the event an anticipated offering is terminated, deferred offering costs will be expensed. As of December 31, 2019, there were no capitalized deferred offering costs on the consolidated balance sheet. As of September 30, 2020, there are $2.1 million of deferred offering costs within prepaid and other current assets on the unaudited condensed consolidated balance sheet.
Concentration of Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and accounts receivable. The Company maintains the majority of its cash balances at multiple financial institutions that management believes are of high-credit quality and financially stable. Cash is deposited with major financial institutions in excess of Federal Deposit Insurance Corporation (“FDIC”) insurance limits. At December 31, 2019 and September 20, 2020 the Company had approximately $23.6 million and $123.9 million, in six and in seven major financial institutions, respectively, in excess of FDIC insurance limitations. The Company provides credit, in the normal course of business, to international and domestic distributors and customers, which are geographically dispersed. The Company attempts to limit its credit risk by performing ongoing credit evaluations of its customers and maintaining adequate allowances for potential credit losses.
F-56
For each significant customer, revenue as a percentage of total revenue for the periods presented and accounts receivable, net as a percentage of total accounts receivable, net as of the periods presented were as follows:
| Revenue | Accounts Receivable, net | |||||||||||||||
| Nine Months Ended September 30, |
December 31, 2019 |
September 30, 2020 |
||||||||||||||
| 2019 | 2020 | |||||||||||||||
| Pfizer Inc. |
* | 14% | * | 52% | ||||||||||||
| BioNTech SE |
* | 10% | * | 14% | ||||||||||||
| Synthorx, Inc. |
* | 10% | * | * | ||||||||||||
| Thermo Fisher Scientific, Inc. |
10% | * | 11% | * | ||||||||||||
| Ultragenyx Pharmaceutical Inc. |
* | * | 10% | * | ||||||||||||
| *less than 10% |
||||||||||||||||
Fair Value of Financial Instruments
The Company defines fair value as the amount that would be received to sell an asset, or paid to transfer a liability, in an orderly transaction between market participants at the measurement date. The Company follows accounting guidance that has a three-level hierarchy for fair value measurements based upon the transparency of inputs to the valuation of the asset or liability as of the measurement date. Instruments with readily available actively quoted prices, or for which fair value can be measured from actively quoted prices in an orderly market, will generally have a higher degree of market price transparency and a lesser degree of judgment used in measuring fair value. The three levels of the hierarchy are defined as follows:
Level 1—Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets;
Level 2—Include other inputs that are directly or indirectly observable in the marketplace; and
Level 3—Unobservable inputs which are supported by little or no market activity.
As of December 31, 2019 and September 30, 2020, the carrying value of current assets and liabilities approximates fair value due to the short maturities of these instruments. The fair values of the Company’s long-term debt approximate carrying value, excluding the effect of unamortized debt discount, as they are based on borrowing rates currently available to the Company for debt with similar terms and maturities (Level 2 inputs).
The fair value of the interest rate caps as of December 31, 2019 and September 30, 2020 were insignificant.
There were no significant assets or liabilities measured at fair value on a recurring basis as of December 31, 2019 or September 30, 2020.
Acquisitions
The Company evaluates acquisitions of assets and other similar transactions to assess whether or not the transaction should be accounted for as a business combination or asset acquisition by first applying a screen test to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets. If the screen is met, the transaction is accounted for as an asset acquisition. If the screen is not met, further determination is required as to whether or not the Company has acquired inputs and processes that have the ability to create outputs which would meet the definition of a business. Significant judgment is required in the application of the screen test to determine whether an acquisition is a business combination or an acquisition of assets.
If the transaction is determined not to be a business combination, it is accounted for as an asset acquisition. For asset acquisitions, a cost accumulation model is used to determine the cost of an asset acquisition. Direct
F-57
transaction costs are recognized as part of the cost of an asset acquisition. The Company also evaluates which elements of a transaction should be accounted for as a part of an asset acquisition and which should be accounted for separately. Consideration deposited into escrow accounts are evaluated to determine whether it should be included as part of the cost of an asset acquisition or accounted for as contingent consideration.
The cost of an asset acquisition, including transaction costs, are allocated to identifiable assets acquired and liabilities assumed based on a relative fair value basis. Goodwill is not recognized in an asset acquisition. Any difference between the cost of an asset acquisition and the fair value of the net assets acquired is allocated to the non-monetary identifiable assets based on their relative fair values. Assets acquired as part of an asset acquisition that are considered to be in-process research and development (IPR&D) are immediately expensed unless there is an alternative future use in other research and development projects.
In addition to upfront consideration, our asset acquisitions may also include contingent consideration payments to be made for future milestone events or royalties on net sales of future products. The Company assesses whether such contingent consideration meets the definition of a derivative. Contingent consideration payments in an asset acquisition not required to be accounted for as derivatives are recognized when the contingency is resolved, and the consideration is paid or becomes payable. Contingent consideration payments required to be accounted for as derivatives are recorded at fair value on the date of the acquisition and are subsequently remeasured to fair value at each reporting date. Contingent consideration payments made are expensed or capitalized as intangible assets, subject to impairment assessments, based on the development and commercialization status of the underlying assets acquired at the time of payment.
The Company classifies cash payments related to purchased intangibles in an asset acquisition, including IPR&D assets, as a cash outflow from investing activities as there is an expectation to generate future income and cash flows from these assets if they can be developed into commercially successful products.
If the acquisition is determined to be a business combination, all tangible and intangible assets acquired, including any IPR&D asset, and liabilities assumed, including contingent consideration, are recorded at their fair value. Goodwill is recognized for any difference between the price of acquisition and our fair value determination. In addition, direct transaction costs in connection with business combinations are expensed as incurred, rather than capitalized.
Emerging Growth Company Status
The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these consolidated financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
Recently Adopted Accounting Pronouncements
In June 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2018-07, Compensation—Stock Compensation (Topic 718):Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-07”). ASU 2018-07 simplifies the accounting for share-based payment transactions in which a grantor acquires goods or services to be used or consumed in operations from a nonemployee. This standard was effective for annual periods beginning after December 15, 2019. The Company’s adoption of this standard as of January 1, 2020 did not have an impact on the unaudited condensed consolidated financial statements.
F-58
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820)—Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”) removed the following disclosure requirements: (1) the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy; (2) the policy for timing of transfers between levels; and (3) the valuation processes for Level 3 fair value measurements. Additionally, this update added the following disclosure requirements: (1) the changes in unrealized gains and losses for the period included in other comprehensive income and loss for recurring Level 3 fair value measurements held at the end of the reporting period; (2) the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. For certain unobservable inputs, an entity may disclose other quantitative information (such as the median or arithmetic average) in lieu of the weighted average if the entity determines that other quantitative information would be a more reasonable and rational method to reflect the distribution of unobservable inputs used to develop Level 3 fair value measurements. The Company’s adoption of this standard as of January 1, 2020 did not have an impact on the unaudited condensed consolidated financial statements.
In December 2019, the FASB issued Accounting Standard Update No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (ASU 2019-12), which eliminates certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also clarifies and simplifies other aspects of the accounting for income taxes. This guidance is effective for fiscal years beginning after December 31, 2021, and interim periods within fiscal years beginning after December 15, 2022. Early adoption is permitted. The Company early adopted ASU 2019-12 effective January 1, 2020. ASU 2019-12 removes the exception to the incremental approach for intra-period tax allocation in the event of a loss from continuing operations and income or gain from other items such as other comprehensive income. The exception previously resulted in allocating a tax benefit to continuing operations and tax expense to other items, even when tax expense may have been zero. Under the simplification, no tax expense or benefit will be recorded to continuing operations. The Company has an immaterial minimum state tax liability in California and no franchises tax liability. These amounts were recorded above-the-line prior to adoption of ASU 2019-12. ASU 2019-12 requires non-income tax-based state franchise taxes to be recorded above-the-line. There is no impact on the Company’s condensed consolidated financial statements for this amendment under ASU 2019-12. The other provisions within ASU 2019-12 are not applicable to the Company.
In July 2017, the FASB issued ASU 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480) and Derivatives and Hedging (Topic 815): I. Accounting for Certain Financial Instruments with Down Round Features; II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception (“ASU 2017-11”). Part I of this update addresses the complexity of accounting for certain financial instruments with down round features. Down round features are features of certain equity-linked instruments (or embedded features) that result in the strike price being reduced on the basis of the pricing of future equity offerings. Current accounting guidance creates cost and complexity for entities that issue financial instruments (such as warrants and convertible instruments) with down round features that require fair value measurement of the entire instrument or conversion option. Part II of this update addresses the difficulty of navigating Topic 480, Distinguishing Liabilities from Equity, because of the existence of extensive pending content in the FASB Accounting Standards Codification. This pending content is the result of the indefinite deferral of accounting requirements about mandatorily redeemable financial instruments of certain nonpublic entities and certain mandatorily redeemable noncontrolling interests. The amendments in Part II of this update do not have an accounting effect. The amendments in Part I of this update were effective for fiscal years beginning after December 15, 2019. The Company’s adoption of Part I of this standard on January 1, 2020 did not have an impact on the unaudited condensed consolidated financial statements.
Recent Issued Accounting Pronouncements Not Yet Adopted
In February 2016, the FASB issued ASU No. 2016-02, Leases (“Topic 842”), which supersedes the guidance in ASC 840, Leases. The new standard, as amended by subsequent ASUs on Topic 842 and recent
F-59
extensions issued by the FASB in response to COVID-19, requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months regardless of their classification. Leases with a term of 12 months or less will be accounted for similar to existing guidance for operating leases today. As a result of the Company having elected the extended transition period for complying with new or revised accounting standards pursuant to Section 107(b) of the JOBS Act, and assuming the Company continues to be considered an Emerging Growth Company, Topic 842 will be effective for the Company on January 1, 2022. The Company has not yet determined the full effects of Topic 842 on its unaudited condensed consolidated financial statements but does expect that it will result in a substantial increase in its long-term assets and liabilities and enhanced disclosures.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments which has been subsequently amended (“ASU 2016-13”). ASU 2016-13 revises the measurement of credit losses for most financial instruments measured at amortized cost, including trade receivables, from an incurred loss methodology to an expected loss methodology which results in earlier recognition of credit losses. Under the incurred loss model, a loss is not recognized until it is probable that the loss-causing event has already occurred. The new standard introduces a forward-looking expected credit loss model that requires an estimate of the expected credit losses over the life of the instrument by considering all relevant information including historical experience, current conditions, and reasonable and supportable forecasts that affect collectability. In addition, this standard also modifies the impairment model for available-for-sale debt securities, which are measured at fair value, by eliminating the consideration for the length of time fair value has been less than amortized cost when assessing credit loss for a debt security and provides for reversals of credit losses through income upon credit improvement. ASU 2016-13, is effective for the Company’s January 1, 2023, with early adoption permitted. The Company is currently assessing the impact of adopting this standard on its unaudited condensed consolidated financial statements and disclosures.
In August 2018, the FASB issued ASU No. 2018-15, Intangibles—Goodwill and Other—Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract. The ASU aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. This new standard also requires customers to expense the capitalized implementation costs of a hosting arrangement that is a service contract over the term of the hosting arrangement. This ASU is effective for years beginning after December 15, 2020, with early adoption permitted. The Company has not yet determined the potential effects of this ASU on its consolidated financial statements.
In January 2017, the FASB adopted ASU 2017-04, Intangibles-Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment (“ASU 2017-04”) which simplifies how an entity is required to test goodwill for impairment by eliminating Step 2 from the goodwill impairment test. Step 2 measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of that goodwill. The implied fair value for a reporting unit is determined in the same manner as the amount of goodwill recognized in a business acquisition of the reporting unit. Under the amendments in ASU 2017-04, an entity shall recognize an impairment charge for the amount by which the carrying amount of a reporting unit exceeds its fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. The updated guidance requires adoption on a prospective basis. ASU 2017-04 is currently effective for the Company beginning January 1, 2022. Early adoption is permitted. The adoption of this standard update is not expected to have a material impact on our unaudited condensed consolidated financial statements; however, any goodwill impairment losses recognized subsequent to adoption will be measured following the updated standard.
F-60
2. Acquisitions
MockV
In March 2020, the Company acquired all of the outstanding shares of MockV Solutions, Inc. (“MockV”), a private entity, for $3.0 million, inclusive of acquisition costs of $0.2 million. The MockV technology acquired is a novel, proprietary viral clearance prediction tool that includes a non-infectious “mock virus particle” mimicking the physicochemical properties of live virus that may be present endogenously in the drug substance or introduced during bioproduction and will expand the Company biologics safety testing offerings. The transaction was accounted for as an asset acquisition as the acquired set of assets and activities did not meet the definition of a business. In connection with this acquisition, the Company acquired developed technology, an in-process research and development asset (“IPR&D”), an assembled workforce, and an insignificant amount of working capital balances. The relative fair value attributed to the acquired developed technology, assembled workforce, and working capital balances was insignificant. The IPR&D acquired was allocated a value of $2.9 million and the Company recognized a charge of $2.9 million related to the IPR&D as a component of research and development on the condensed consolidated statement of operations because the technology had not yet reached technological feasibility and had no alternative future use. The Company must also make contingent cash payments (the “Earn-Outs”) of up to $9.0 million to the sellers of MockV based upon the achievement of long-term revenue targets. The Earn-Outs were determined to be contingent consideration that was not subject to derivative accounting and will be recognized when the contingency is resolved, and the consideration becomes paid or payable.
As the Company had no tax basis in the acquired IPR&D asset, and the acquired IPR&D asset was expensed prior to the measurement of any deferred taxes, no deferred taxes were recognized for the initial transaction.
3. Goodwill and Intangible Assets
There were no changes to the carrying value of goodwill as of December 31, 2019 and September 30, 2020. Total amortization expense related to intangible assets was $15.2 million for the nine months ended September 30, 2019 and 2020.
The following is a summary of the Company’s goodwill by segment (in thousands):
| Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Total | |||||||||||||
| December 31, 2019 |
$ | 32,838 | $ | 119,928 | $ | 71,509 | $ | 224,275 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| September 30, 2020 |
$ | 32,838 | $ | 119,928 | $ | 71,509 | $ | 224,275 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The components of finite-lived intangible assets and accumulated amortization are as follows:
| As of December 31, 2019 | ||||||||||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Estimated Useful Life |
Weighted Average Remaining Amortization Period |
||||||||||||||||
| (in thousands) | (in years) | (in years) | ||||||||||||||||||
| Trade Names |
$ | 11,490 | $ | 4,093 | $ | 7,397 | 5-15 | 7.3 | ||||||||||||
| Patents and Developed Technology |
169,313 | 40,134 | 129,179 | 5-14 | 10.5 | |||||||||||||||
| Customer Relationships |
83,290 | 22,013 | 61,277 | 10-14 | 9.8 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 264,093 | $ | 66,240 | $ | 197,853 | 10.1 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
F-61
| As of September 30, 2020 | ||||||||||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Estimated Useful Life |
Weighted Average Remaining Amortization Period |
||||||||||||||||
| (in thousands) | (in years) | (in years) | ||||||||||||||||||
| Trade Names |
$ | 11,490 | $ | 5,061 | $ | 6,429 | 5-15 | 6.6 | ||||||||||||
| Patents and Developed Technology |
169,404 | 49,581 | 119,823 | 5-14 | 9.7 | |||||||||||||||
| Customer Relationships |
83,323 | 26,754 | 56,568 | 10-14 | 9.1 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 264,217 | $ | 81,396 | $ | 182,820 | 9.4 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
The Company recognized $9.1 million and $9.5 million of amortization expense from intangible assets directly linked with revenue generating activities within cost of revenue in the consolidated statement of operations for the nine months ended September 30, 2019 and 2020, respectively. Amortization expense for intangible assets that are not directly related to sales generating activities of $6.0 million and $5.7 million, respectively, was recorded to selling, general and administrative expenses for each of the nine months ended September 30, 2019 and 2020.
| 2020 (remaining three months) |
$ | 5,158 | ||
| 2021 |
20,079 | |||
| 2022 |
19,428 | |||
| 2023 |
19,230 | |||
| 2024 |
19,230 | |||
| Thereafter |
99,695 | |||
|
|
|
|||
| Total estimated amortization expense |
$ | 182,820 | ||
|
|
|
4. Fair Value Measurements
The following table provides a reconciliation of liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) for the nine months ended September 30, 2019 (in thousands):
| Contingent Consideration |
||||
| Balance at December 31, 2018 |
$ | 3,678 | ||
| Change in fair value |
241 | |||
| Settlement |
(2,000 | ) | ||
|
|
|
|||
| Balance at September 30, 2019 |
$ | 1,919 | ||
|
|
|
|||
During the nine-month period ended September 30, 2019, the first $2.0 million due under the 2017 asset purchase agreement was paid to Glen Research. During the last quarter of calendar year 2019, upon the achievement of the maximum liability threshold, as defined by the agreement, of $4.0 million, the total contingent purchase consideration was no longer based on significant unobservable inputs and was reclassified out of Level 3 fair value hierarchy to accrued expenses.
F-62
5. Balance Sheet Components
Inventory
Inventory consists of the following at December 31, 2019 and September 30, 2020 (in thousands):
| December 31, 2019 |
September 30, 2020 |
|||||||
| Raw materials |
$ | 5,037 | $ | 9,392 | ||||
| Work in process |
6,083 | 13,676 | ||||||
| Finished goods |
3,082 | 3,518 | ||||||
|
|
|
|
|
|||||
| $ | 14,202 | $ | 26,586 | |||||
|
|
|
|
|
|||||
Accrued expenses
Accrued expenses consist of the following at December 31, 2019 and September 30, 2020 (amount in thousands):
| December 31, 2019 |
September 30, 2020 |
|||||||
| Employee related |
$ | 7,660 | $ | 14,208 | ||||
| Professional services |
3,199 | 3,537 | ||||||
| Sales and use tax liability |
2,907 | 3,237 | ||||||
| Accrued interest |
475 | 1,875 | ||||||
| Accrued construction costs |
1,165 | 4,661 | ||||||
| Consideration payable |
2,000 | — | ||||||
| Current portion of deferred gain on sale and leaseback transaction |
— | 1,118 | ||||||
| Other |
1,109 | 5,047 | ||||||
|
|
|
|
|
|||||
| Total accrued expenses |
$ | 18,515 | $ | 33,683 | ||||
|
|
|
|
|
|||||
6. Commitments and Contingencies
Lease Commitments
The Company leases five facilities, including office, laboratory and manufacturing space under long-term non-cancelable operating leases. The leased facilities have initial terms of two to twelve years, and two leases have multiple five-year renewal terms and the other leases having three-year and five-year renewal terms. The Company also has capital leases for office equipment with initial terms of two to three years expiring in 2023.
Rent expense for nine months ended September 30, 2019 and 2020 was approximately $1.8 million and $2.3 million, respectively. For the nine months ended September 30, 2020, reported rent expense is net of approximately $1.1 million of deferred gain recognized over the life of the lease associated with the Company’s sale leaseback arrangement for its Burlingame, California facility.
In January 2020, the Company completed the sale of land, building and related building improvements specific to its facility in Burlingame, California for approximately $34.5 million in cash. Simultaneously, with the close of the transaction, the Company leased the property for a two-year period, resulting in a total of $3.3 million in new lease obligations through December 31, 2021. The Company’s sale of the building and immediate leaseback of the facility qualified for sale-leaseback accounting. The lease was evaluated and classified as an operating lease. Given the Company was considered to retain more than a minor part but less than substantially all of the use of the property, the present value of the minimum lease payment over the lease term of
F-63
$3.1 million reduced the gain on the sale of the asset and will be recognized as a reduction of rent expense over the life of the lease. Net of the $3.1 million in deferred gain, the Company recognized a net gain on the sale and leaseback transaction of $19.0 million during the nine months ended September 30, 2020. In August 2020, the Company executed a six-month extension for the leased property, including escalating rent payments, with total incremental lease payments associated with the extension of $1.8 million. The unamortized deferred gain at the time of the modification, approximating $2.0 million, will be amortized on a prospective basis over the extended lease term. Upon execution of the amendment inclusive of escalating rent payments, expense is being recognized on a straight-line basis and the difference between the recognized rent expense and the amounts paid under the lease are being recorded as deferred rent included in other short-term and long-term liabilities on the condensed consolidated balance sheet as of September 30, 2020.
In July 2018, the Company entered into a lease for a new manufacturing facility (the “San Diego Facility Lease”). The lease included tenant improvement provisions for construction prior to occupancy. Construction on this new manufacturing facility began in 2018 and Company evaluated the extent of its financial and operational involvement in the tenant improvements of the new facility related to the San Diego Facility Lease to determine whether it was considered the owner of the construction project under GAAP. The Company concluded that it was deemed to be the owner of the facility for accounting purposes (even though it did not meet the definition for legal purposes) during the construction period. In 2019, upon completion of the construction, the Company evaluated the lease and concluded that the completed construction project failed to qualify for sale and leaseback accounting primarily due to the $8.0 million of non-recourse financing the Company provided to the lessor by reimbursing them for the $8.0 million of construction costs. The Company has accounted for the lease as a financing lease transaction. The leased building and related improvements remain on the Company’s balance sheet as of December 31, 2019 and rental payments associated with the San Diego Facility Lease have been allocated to operating lease expense for the ground underlying the leased building and principal and interest payments on the lease facility financing obligation. The Company recorded the fair value of the building asset and improvements, which was estimated to be $59.0 million and the related lease facility financing obligation of $51.2 million. The difference between the gross asset value and the lease facility financing obligation represents the approximate $8.0 million of building improvement costs reimbursed by the Company.
In September 2020, the Company amended its San Diego Facility lease agreement to provide for additional manufacturing and office space. The amended lease agreement provides for tenant improvements for construction prior to occupancy of $2.7 million, rent concessions, and escalating rent payments over the life of the lease which now expires in May 2023. The total future minimum lease payments under the amended lease agreement are $57.1 million, with an option to renew subject to certain conditions. Similar to the original lease, once construction is completed on the expansion, the 2020 amended lease is being accounted for as an increase to the financing lease transaction with rental payment allocated between principal and interest on the lease facility financing obligation. As of September 30, 2020, the anticipated tenant and the tenant improvement allowance has been recorded as a component of the lease facility financing obligation and receivable from lessor funded financing within prepaid and other current assets on the September 30, 2020 condensed consolidated balance sheet. Additionally, during 2020, the Company incurred building improvement costs for incremental improvement costs for the initially leased space. As of September 30, 2020, the Company has recognized $16.6 million and $4.7 million in construction in progress and accrued expenses, respectively, within the condensed consolidated balance sheet.
The Company recognizes payments under the lease agreement as a reduction of the lease facility financing obligation using the effective interest method and the ground rent as operating lease expense as reflected in the schedule below. The allocation of the San Diego Facility Lease payment to ground lease rent expense and principal and interest expense on the lease facility financing obligation was estimated using income and market approaches that utilized comparable observable sales for similar assets, land capitalization rates, and an estimate of the Company’s incremental borrowing rate (Level 2 and Level 3 inputs). The fair value of the leased property, less its expected residual value, is depreciated over the term of the lease. At the conclusion of the lease term, the Company will de-recognize both the then carrying values of this asset and lease facility financing obligation with
F-64
any differences between the book value of the asset and remaining lease facility financing obligation being recognized in operations at that time. For the Company’s arrangement for the San Diego Facility Lease, these differences are expected to be immaterial. Payments on the San Diego Facility Lease obligation during the nine months ended September 30, 2019 and 2020 were approximately $0.9 million and $1.7 million, respectively. For the nine months ended September 30, 2019 and 2020, the Company recognized rent expense associated with the ground lease for the San Diego Facility Lease of approximately $0.2 million and $0.6 million in the condensed consolidated statement of operations.
As of September 30, 2020, minimum annual payments under the Company’s non-cancelable lease agreements, capital lease agreements, and lease financing obligations are as follows (in thousands):
| Capital Leases |
Lease Facility Financing Obligations |
Operating Leases |
||||||||||
| 2020 (remaining three months) |
$ | 12 | $ | 560 | $ | 808 | ||||||
| 2021 |
52 | 4,087 | 2,718 | |||||||||
| 2022 |
21 | 4,351 | 2,942 | |||||||||
| 2023 |
1 | 4,670 | 1,203 | |||||||||
| 2024 |
— | 4,756 | 1,234 | |||||||||
| 2025 and beyond |
— | 26,781 | 5,579 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total minimum payments |
86 | 45,205 | $ | 14,484 | ||||||||
|
|
|
|||||||||||
| Less: amount representing interest |
(20 | ) | (28,136 | ) | ||||||||
|
|
|
|
|
|||||||||
| Present value of future minimum lease payments |
66 | 17,069 | ||||||||||
| Residual value of lease facility financing obligation |
— | 39,558 | ||||||||||
| Less: short-term capital lease and lease facility financing obligations |
(43 | ) | (187 | ) | ||||||||
|
|
|
|
|
|||||||||
| Long-term capital lease and lease facility financing obligations |
$ | 23 | $ | 56,440 | ||||||||
|
|
|
|
|
|||||||||
Operating leases in the table above includes future minimum lease payments for the ground lease for the
Southport North Carolina facility and San Diego facility leases.
Legal Proceedings
The Company is involved in various legal proceedings arising in the normal course of business. The Company accrues for a loss contingency when it determines that it is probable, after consultation with counsel, that a liability has been incurred and the amount of such loss can be reasonably estimated. The Company believes that the results of any such contingencies, either individually or in the aggregate, will not have a material adverse effect on the Company’s financial position, results of operations or cash flows.
Indemnification Agreements
In the ordinary course of business, we may provide indemnification of varying scope and terms to vendors, lessors, customers and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from intellectual property infringement claims made by third parties. These indemnities include indemnities to our directors and officers to the maximum extent permitted under applicable state laws. The maximum potential amount of future payments that we could be required to make under these indemnification agreements is, in many cases, unlimited. We have not incurred any material costs as a result of such indemnifications and are not currently aware of any indemnification claims.
F-65
7. Long-Term Debt
As of December 31, 2019 and September 30, 2020, the Maravai Intermediate Holdings, LLC (“Intermediate”), a wholly-owned subsidiary of the Company, along with its subsidiaries (the “Borrowers”), had a First Lien Credit Agreement (the “First Lien Agreement”) and a Second Lien Credit Agreement (the “Second Lien Credit Agreement”) (collectively referred to as the “First and Second Lien Credit Agreements”) with lending institutions for term-loan borrowings (“First Lien Term Loan”) totaling $250.0 million and for loans (“Second Lien Loan”) totaling $100.0 million, (collectively referred to as the “First and Second Lien Loans”). The First Lien Credit Agreement also provides for a revolving credit facility (the “Revolving Credit Facility”) of $50.0 million for letters of credit and loans to be used for working capital and other general corporate financing purposes, of which $15.0 million was drawn down in March 2020 to provide financing for the acquisition of MockV and other operating uses. Repayment of the borrowings on the Revolving Credit Facility are due upon maturity in August 2025. Borrowings under the First and Second Lien Loans are unconditionally guaranteed by the Company, immediate parent of Intermediate, along with the existing and future domestic subsidiaries of the Company (subject to certain exceptions) as specified in the respective guaranty agreements, and are secured by a lien and security interest in substantially all of the assets of existing and future domestic subsidiaries of the Company that are loan parties.
At December 31, 2019 and September 30, 2020, the interest rate on the First Lien Term Loan was 6.062% and 5.250%, respectively. At December 31, 2019 and September 30, 2020, the interest rate on the Second Lien Loan was 9.734% and 9.000%, respectively
The First Lien Credit Agreement also requires mandatory prepayments to be calculated in 2019 upon certain excess cash flow as defined in the terms of the agreement to be paid beginning in 2020. As of September 30, 2020, no mandatory prepayment was required.
The Borrowers were in compliance with all of their debt covenants under the First and Second Lien Agreements as of September 30, 2020 and there were no events of default for the nine months ended September 30, 2020.
The Company’s total debt at December 31, 2019 and September 30, 2020 consisted of (in thousands):
| 2019 | 2020 | |||||||
| First Lien Term Loan |
$ | 246,875 | $ | 245,000 | ||||
| Second Lien Loan |
100,000 | 100,000 | ||||||
| Revolving Credit Facility |
— | 15,000 | ||||||
| Unamortized debt issuance costs |
(9,592 | ) | (8,296 | ) | ||||
|
|
|
|
|
|||||
| Total long-term debt |
337,283 | 351,704 | ||||||
| Less: current portion |
(2,500 | ) | (2,500 | ) | ||||
|
|
|
|
|
|||||
| Total long-term debt, less current portion |
$ | 334,783 | $ | 349,204 | ||||
|
|
|
|
|
|||||
As of September 30, 2020, the aggregate future principal maturities of the Company’s debt obligations for each of the next five years, based on contractual due dates, are as follows (in thousands):
| 2020 (remaining three months) |
$ | 650 | ||
| 2021 |
2,500 | |||
| 2022 |
2,500 | |||
| 2023 |
2,500 | |||
| 2024 |
2,500 | |||
| Thereafter |
349,350 | |||
|
|
|
|||
| Total debt |
$ | 360,000 | ||
|
|
|
F-66
8. Member’s Equity
During the nine months ended September 30, 2020, $0.3 million of tax distributions were made to certain holders of MLSC Incentive Units. No distributions to the holders of MLSC Incentive Units were made during the nine months ended September 30, 2019. During the nine months ended September 30, 2019 and 2020, no distributions were made to Class A or Class B preferred unitholders of MLSC or to the Company’s member.
MLSC Incentive Units
The Company’s majority-owned subsidiary, MLSC, is authorized and at the discretion of the MLSC Board, under its Limited Liability Company Agreement (“MLSC LLC Agreement”), as amended in October 2016, to issue common units that can be designated as Incentive Units. The Incentive Units may be subject to either a combination of service, market or performance vesting conditions. Vested Incentive Units are treated as common units for purposes of distributions.
For MLSC Incentive Units that remain subject to performance conditions at December 31, 2019, the Company concluded that it was not yet probable that the performance conditions would be met. Accordingly, the Company has not recognized any compensation expense in the accompanying consolidated statements of operations and comprehensive loss for Incentive Units that include a performance condition.
No MLSC Incentive Units were granted in the nine months ended September 30, 2020.
In September 2020, the Company entered into agreements (the “Repurchase Agreements”) to repurchase all outstanding MLSC Incentive Units, including the 1,500,000 MLSC Incentive Units held by the President of Cygnus Technologies, LLC, (“Cygnus”) a subsidiary of MLSC. As part of the Repurchase Agreements, the Company accelerated the vesting of all 855,667 remaining unvested time-based MLSC Incentive Units and also removed the performance condition associated with 249,000 performance-based MLSC Incentive Units. The Company has accounted for the acceleration of the vesting on the time-based MLSC Incentive Units under settlement accounting, which resulted in the recognition of all remaining unrecognized compensation cost as of the dates of the Agreements. Such accelerated compensation cost totaled $0.4 million. The Company has accounted for the removal of the performance condition and the ensuing acceleration of the vesting of the 249,000 performance-based MLSC Incentive Units as an improbable-to-probable modification, which provides for premeasurement of the related compensation cost at the fair value of these incentive units as of the date of the Agreements. The total compensation cost recognized in September 2020 for these performance-based MLSC Incentive Units approximated $0.8 million. The Company paid $9.1 million to settle the Repurchase Agreements in October 2020 and recorded a repurchase liability for incentive units of $9.1 million at September 30, 2020 on the condensed consolidated balance sheet.
Unit-based compensation expense for the nine months ended September 30, 2019 and September 30, 2020 was approximately $1.2 million and $2.9 million, respectively.
As of December 31, 2019, there were 2,745,000 MLSC Incentive Units outstanding, of which 821,400 remained unvested. There were no outstanding MLSC Incentive Units outstanding as of September 30, 2020.
MLSH 1 Incentive Units
The Company has entered into agreements with certain executives and board members whereby those employees and board members were granted incentive units in MLSH 1, the Company’s parent and sole member (“MLSH 1 Incentive Units”). All MLSH 1 Incentive Unit awards are subject to a market condition which is subject to the achievement of a certain investment return threshold that increases on a compounding basis annually and a service condition subject to their continued employment. Certain MLSH 1 Incentive Unit awards also contain performance conditions tied to the consummation of a business acquisition. Other MLSH 1 Incentive
F-67
Unit awards contain a performance condition tied to the achievement of certain cash distribution multiples. The fair value of MLSH 1 Incentive Unit awards is measured at the grant date and is recognized as expense over the requisite service period for the awards. For MLSH 1 Incentive Units that remain subject to performance conditions at December 31, 2019 and September 30, 2020, the Company concluded that it was not yet probable that the performance conditions would be met. Accordingly, the Company has not recognized any compensation expense in the accompanying consolidated statements of operations and comprehensive loss for MLSH 1 Incentive Units that include a performance condition.
MLSH 1 Incentive Unit award activity during the nine-month period ended September 30, 2020 is as follows:
| Number of Unvested MLSH 1 Incentive Unit awards |
Weighted Average Grant Date Fair Value |
|||||||
| Balance as of December 31, 2019 |
787,100 | $ | 11.53 | |||||
| MLSH 1 Incentive Unit awards granted |
62,000 | 42.11 | ||||||
| MLSH 1 Incentive Unit awards vested |
(143,600 | ) | 7.15 | |||||
|
|
|
|||||||
| Balance as of September 30, 2020 |
705,500 | $ | 13.44 | |||||
|
|
|
|||||||
The following table sets forth the compensation expense related to both the MLSC and MLSH 1 Incentive Units included in the accompanying consolidated statements of operations (in thousands):
| Nine Months Ended September 30, |
||||||||
| 2019 | 2020 | |||||||
| Cost of revenues |
$ | 16 | $ | 11 | ||||
| Research and development |
157 | 732 | ||||||
| Selling, general and administrative |
990 | 2,190 | ||||||
|
|
|
|
|
|||||
| $ | 1,163 | $ | 2,933 | |||||
|
|
|
|
|
|||||
As of September 30, 2020, there was $8.5 million of unrecognized compensation cost associated with the total of all MLSH 1 Incentive Units awards, including those subject to a performance condition.
9. Repurchase Liability for Noncontrolling Interests
The noncontrolling interest in MLSC, the parent of Cygnus, as of December 31, 2019 represented equity interest that was retained by the members of MLSC prior to its acquisition by the Company in October 2016. The President of Cygnus and his affiliated entity, considered to be related parties, were the owners of the noncontrolling interests.
In September 2020, the Company and MLSH 1 entered into a Sale and Rollover Agreement with the President of Cygnus Technologies and his affiliated entity (collectively, the “Investors”) to purchase 43,264 MLSC Class B preferred units and 18,387,206 MLSC common units held by the Investors for approximately $120.0 million, at the notice of the Company, but no later than two days following the occurrence of an IPO.
In addition, the Sale and Rollover Agreement provides that the remaining 16,736 MLSC Class B preferred units and 7,112,794 MLSC common units held by the Investors are subject to exchange into a variable number of common units of MLSH 1 with a fixed total value of approximately $46.4 million (the “Exchange”). The Exchange shall take place immediately prior to, and is conditioned upon, the occurrence of an IPO. If the Exchange is not consummated prior to January 2021, the Investors, other than the President of Cygnus
F-68
Technologies, have the right to request a reallocation (a “Reallocation Request Notice”) of the amount of their MLSC Class B preferred and common units subject to repurchase for cash and those subject to the Exchange. If a Reallocation Request Notice is not accepted and a cash repurchase does not occur within 14 days of receipt of a Reallocation Request Notice, then this subset of the Investors has the right to terminate the Sale and Rollover Agreement.
The provisions of the Sale and Rollover Agreement are considered to be freestanding obligations of the Company to redeem noncontrolling interests in MLSC. Based on the distinguishing liabilities from equity guidance, the obligation to redeem these MLSC Class B preferred and common units for cash or a variable number of shares with a fixed monetary value was recognized as a liability. The difference between the consideration to be paid to the Investors associated with the noncontrolling interests of $166.4 million and the carrying amount of the noncontrolling interests in MLSC of $5.3 million, as of the date of the Sale and Rollover Agreement, was recorded as a $161.1 million reduction in contributed capital in the consolidated balance sheet as of September 30, 2020. The outstanding liability associated with the repurchase and Exchange of the noncontrolling interests of $166.4 million has been reflected as a current liability on the condensed consolidated balance sheets as of September 30, 2020. In October 2020, the Company repurchased $120.0 million of the MLSC Class B preferred and common units for cash and the remaining $46.4 million for MLSC Class B preferred and common units remains outstanding will be settled on the occurrence of the Exchange.
10. Income Taxes
The Company and a number of its subsidiaries are treated as flow-through entities for federal income tax purposes. The income or loss generated by these entities are not taxed at the LLC level. As required by U.S. tax law, income or loss generated by these LLCs flows through to MLSH 1, the Company’s sole member. As such, the Company’s income tax provision consists solely of the activities of its taxable subsidiaries which are taxed as corporations for federal income tax purposes.
The Company’s effective income tax rate was (12.1%) and 23.7% for the nine months ended September 30, 2019 and 2020, respectively. The change in the Company’s effective income tax rate for the nine months ended September 30, 2019 compared to the nine months ended September 30, 2020 was primarily driven by an increase in the Company’s pre-tax book income and the release of a valuation allowance on the Internal Revenue Code 163(j) interest expense carryforward. The effective tax rate for the nine months ended September 30, 2019 differed from the U.S. federal statutory rate of 21% primarily due to recording a valuation allowance against interest expense deferred for tax purposes which did not meet the more likely than not threshold for realizability. The effective tax rate tax rate for the nine months ended September 30, 2020 differed from the US federal statutory rate of 21% primarily as a result of the impact of state taxes offset by the release of valuation allowance on its interest expense carryforward due to the increase in limitation provided by the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”).
The CARES Act was signed into law on March 27, 2020. The CARES Act provides various tax provisions and other stimulus measures, including allowing taxpayers to deduct more business interest expense for taxable years beginning in either 2019 or 2020 by increasing the limitation from 30% to 50% of adjusted taxable income. During the nine months ended September 30, 2020, the Company released $1.3 million of valuation allowance on its interest expense carryforward due to the increase in limitation provided by the CARES Act.
As of September 30, 2020, uncertain tax positions were insignificant, and the Company does not anticipate material changes within the next 12 months.
11. Net Income (Loss) per Common Unit Attributable to the Member of Maravai
Net income (loss) per common unit attributable to our member for the nine-month periods ended September 30, 2019 and 2020 is based on the weighted average number of common units outstanding during the
F-69
period. The members’ equity of MLSC is comprised of Class A and Class B preferred units, MLSC Incentive Units and common units, each with participation rights. The MLSC preferred units are entitled to cumulative dividends of 8.0% compounded annually, up to an additional 4.0%, also compounded annually, to the extent of remaining unallocated earnings. The preferred unitholders of MLSC are required, however, to share a portion of the additional 4.0% in dividends with the holders of MLSC Incentive Units based on a formula defined in the MLSC LLC Agreement. The Company determined that vested MLSC Incentive Units and MLSC Class A and B Preferred Units are participating securities under the two-class method at the MLSC subsidiary level, however, they do not have a contractual obligation to share in losses, and therefore no undistributed losses have been allocated to them. Undistributed losses of MLSC are allocated on a pro rata basis between the Company and the noncontrolling interests based on their respective weighted average outstanding common units owned during each period. MLSH 1 Incentive Units are granted by the parent of the Company, and as a result, do not represent potential common units of the Company.
Diluted net income per common unit attributable to our member is computed by adjusting the weighted-average number of common units outstanding to give effect to any potential common units, if dilutive. There were no potential common units of the Company outstanding during the periods presented. The Company has issued potentially dilutive instruments in the form of MLSC Incentive Units granted to employees and officers of MLSC. As a result of a net loss for MLSC, after giving effect to the Class A and Class B preferred unit dividends, in each of the nine months ended September 30, 2019 and 2020, these MLSC Incentive Units were antidilutive.
The following table sets forth the computation of basic and diluted net income (loss) per common unit attributable to our member for the nine months ended September 30, 2019 and 2020 (in thousands, except units and per unit amounts):
| Nine Months Ended September 30, | ||||||||
| 2019 | 2020 | |||||||
| Net income (loss) per common unit—basic and diluted: |
||||||||
| Net income |
$ | 249 | $ | 64,344 | ||||
| Less: preferred unit dividends attributable to noncontrolling interests |
(4,248 | ) | (14,805 | ) | ||||
| Add: loss attributable to common noncontrolling interests |
1,906 | 3,993 | ||||||
|
|
|
|
|
|||||
| Net (loss) income attributable to the Company common unitholder |
$ | (2,093 | ) | $ | 53,532 | |||
|
|
|
|
|
|||||
| Weighted average common units outstanding |
253,916,941 | 253,916,941 | ||||||
|
|
|
|
|
|||||
| Net (loss) income per common unit—basic and diluted: |
$ | (0.01 | ) | $ | 0.21 | |||
In September 2020, the Company entered into a Sale and Rollover Agreement that provides for the repurchase of a majority of the outstanding Class B preferred units as well as an agreement to exchange the remaining Class B preferred and common units into a variable number of MLSH 1 units contingent upon the occurrence of an IPO (see Note 9). Included in the preferred unit dividends attributable to noncontrolling interests line item is a $10.2 million deemed dividend representing the excess of the fair value of the Class B preferred units, determined as of the date of the Sale and Rollover Agreement, over their related carrying value. As in September 2020, the Company also agreed to repurchase all MLSC Incentive Units, however, such incentive units remained outstanding as of September 30, 2020, and have the potential to be dilutive to earnings per unit as they continue to have participation rights until they are repurchased (see Note 8).
F-70
The following securities have been excluded from the calculations of diluted net loss per common unit for the nine months ended September 30, 2019 and 2020 because their inclusion would be antidilutive:
| Nine Months Ended September 30, |
||||||||
| 2019 | 2020 | |||||||
| Time-based incentive units |
2,896,000 | 2,896,000 | ||||||
| Performance-based incentive units |
249,000 | 249,000 | ||||||
|
|
|
|
|
|||||
| 3,145,000 | 3,145,000 | |||||||
|
|
|
|
|
|||||
12. Segments
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. When determining the reportable segments, the
Company aggregated operating segments based on their similar economic and operating characteristics. Segment results are presented in the same manner as we present our operations internally to make operating decisions and assess performance. The Company’s financial performance is reported in three segments. A description of each segment follows:
| • | Nucleic Acid Production: focuses on the manufacturing and sale of highly modified nucleic acids products to support the needs of customers’ research, therapeutic and vaccine programs. This segment also provides research products for labeling and detecting proteins in cells and tissue samples. |
| • | Biologics Safety Testing: focuses on manufacturing and selling biologics safety and impurity tests and assay development services that are utilized by our customers in their biologic drug manufacturing spectrum. |
| • | Protein Detection: focuses on manufacturing and selling labeling and visual detection reagents to scientific research customers for their tissue-based protein detection and characterization needs. |
The Company has determined that adjusted earnings before interest, tax, depreciation, and amortization (“Adjusted EBITDA”) is the profit or loss measure that the CODM uses to make resource allocation decisions and evaluate segment performance. Adjusted EBITDA assists management in comparing the segment performance on a consistent basis for purposes of business decision-making by removing the impact of certain items that management believes do not directly reflect the core operations and, therefore, are not included in measuring segment performance.
The Company defines Adjusted EBITDA as net income before interest, taxes, depreciation and amortization, certain non-cash items, and other adjustments that we do not consider in our evaluation of ongoing operating performance from period to period. Corporate costs are managed on a standalone basis and not allocated to segments.
F-71
Following is financial information relating to the operating segments (in thousands):
| As of and for the nine months ended |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Eliminations | Total | ||||||||||||||||||
| Revenue |
$ | 54,867 | $ | 32,819 | $ | 19,494 | $ | — | $ | — | $ | 107,180 | ||||||||||||
| Adjusted EBITDA |
$ | 18,551 | $ | 26,961 | $ | 10,841 | $ | (8,203 | ) | $ | — | $ | 48,150 | |||||||||||
| As of and for the nine months ended |
Nucleic Acid Production |
Biologics Safety Testing |
Protein Detection |
Corporate | Eliminations | Total | ||||||||||||||||||
| Revenue |
$ | 129,645 | $ | 40,772 | $ | 16,404 | $ | — | $ | (1,076 | ) | $ | 185,745 | |||||||||||
| Adjusted EBITDA |
$ | 76,130 | $ | 33,571 | $ | 6,960 | $ | (11,597 | ) | $ | (224 | ) | $ | 104,840 | ||||||||||
There was no inter-segment activity for the nine-month period ending September 30, 2019. During the nine-month period ending September 30, 2020, intersegment revenue was $1.1 million. The intersegment sales and the related gross margin on inventory recorded at the end of the period are eliminated for consolidation purposes in the Eliminations column. Internal selling prices for intersegment sales are consistent with the segment’s normal retail price offered to external parties. There was no commission expense recognized for intersegment sales for the nine months ended September 30, 2020. Intersegment revenue represents intersegment revenue between the Nucleic Acid Production and Protein Detection segments.
The Company does not allocate assets to its reportable segments as they are not included in the review performed by the CODM for purposes of assessing segment performance and allocating resources. Excluding approximately $0.3 million associated with a building in the United Kingdom, all of the Company’s long-lived assets are located within the United States.
A reconciliation of Adjusted EBITDA to net income, the most directly comparable GAAP measure, is set forth below:
| Nine Months Ended September 30, |
||||||||
| 2019 | 2020 | |||||||
| Net income |
$ | 249 | $ | 64,344 | ||||
| Add: |
||||||||
| Amortization |
15,118 | 15,156 | ||||||
| Depreciation |
2,331 | 4,756 | ||||||
| Interest expense |
22,240 | 21,934 | ||||||
| Income tax expense |
308 | 2,511 | ||||||
|
|
|
|
|
|||||
| EBITDA |
40,246 | 108,701 | ||||||
| Acquisition contingent consideration |
241 | — | ||||||
| Acquisition integration costs |
4,061 | 3,588 | ||||||
| Amortization of purchase accounting inventory step-up |
1,856 | — | ||||||
| Acquired in-process research and development costs |
— | 2,881 | ||||||
| Unit-based compensation |
1,163 | 2,933 | ||||||
| GTCR management fees |
421 | 555 | ||||||
| Gain on sale and leaseback transaction |
— | (19,002 | ) | |||||
| Merger and acquisition related expenses |
162 | 218 | ||||||
| Financing costs |
— | 4,966 | ||||||
|
|
|
|
|
|||||
| Adjusted EBITDA |
$ | 48,150 | $ | 104,840 | ||||
|
|
|
|
|
|||||
F-72
13. Related Party Transactions
Pursuant to the advisory services agreement with GTCR, LLC, MLSH 1’s majority owner, during both the nine months ended September 30, 2019 and 2020, the Company incurred approximately $0.4 million in management fees to GTCR under an advisory services agreement. During the nine months ended September 30, 2019 and 2020, the Company also incurred out-of-pocket expenses to GTCR of $46,000 and $0.2 million, respectively. As of September 30, 2020, there were no outstanding liabilities to GTCR.
For the nine-month period ending September 30, 2019 and September 30, 2020, the Company paid $0.2 million in lease payments for a leased facility, owned by an entity controlled by a close relative of the President of one of its subsidiaries.
14. Subsequent Events
The Company has evaluated all events occurring through October 29, 2020 (except for the impact of the unit split as discussed in the fifth paragraph of Note 1, as to which the date is November 11, 2020), the date on which the condensed consolidated financial statements were issued, during which time nothing has occurred outside the normal course of business operations that would require disclosure other than the event disclosed below.
In October 2020, Intermediate along with its subsidiaries Vector Laboratories, Inc., TriLink BioTechnologies, LLC and Cygnus (together with Intermediate, the “New Borrowers”), entered into a credit agreement (the “New Credit Agreement”) to refinance existing $400.0 million long-term debt with a new $780.0 million facility which provided for the full repayment of $363.0 million of long-term debt and accrued interest under the First and Second Lien Credit Agreements and Revolving Credit Facility, the payment of the repurchase liability for incentive units and the repurchase liability for noncontrolling interests to be settled in cash of $9.1 million and $120.0 million, respectively, and to allow for a $88.6 million distribution to our member for various incentive unit holders of MLSH 1. The New Credit Agreement provides for a First Lien Term Loan (the “Net First Lien Term Loan”) of $600.0 million, maturing October 2027, and a Revolving Credit Facility (the “Net Revolving Credit Facility”) for up to $180.0 million in funding, which the New Borrowers did not draw against as of the close of refinancing. Borrowings under the New First Lien Term Loan bear interest at 3.25% per annum, allow for prepayment upon achieving specified criteria per the agreement, require mandatory prepayments based upon a calculation of excess of cash flow as defined and applicable, and contain selective financial covenants. Borrowing against the Net Revolving Credit Facility also bears interest at rate of 3.25% per annum and allows the New Borrowers to repay and borrow from time to time until October 2025 at which time all amounts borrowed must be repaid.
F-73

INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth all costs and expenses, other than the underwriting discounts and commissions payable by us, in connection with the offer and sale of the securities being registered. All amounts shown are estimates except for the Securities and Exchange Commission (“SEC”) registration fee and the FINRA filing fee.
| Amount to be Paid | ||||
| SEC registration fee |
$ | 169,378 | ||
| FINRA filing fee |
217,875 | |||
| Nasdaq listing fee |
270,000 | |||
| Advisory fees payable to Perella Weinberg Partners LP |
9,375,000 | |||
| Printing expenses |
750,000 | |||
| Legal fees and expenses |
4,000,000 | |||
| Accounting fees and expenses |
4,200,000 | |||
| Transfer agent fees and registrar fees |
5,000 | |||
| Miscellaneous expenses |
1,380,000 | |||
|
|
|
|||
| Total expenses |
$ | 20,367,253 | ||
|
|
|
|||
Item 14. Indemnification of Directors and Officers
Section 102(b)(7) of the DGCL allows a corporation to provide in its certificate of incorporation that a director of the corporation will not be personally liable to the corporation or its shareholders for monetary damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation will provide for this limitation of liability.
Section 145 of the DGCL (“Section 145”) provides that a Delaware corporation may indemnify any person who was, is or is threatened to be made party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his conduct was illegal. A Delaware corporation may indemnify any persons who are, were or are threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit, provided that such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests, provided that no indemnification is permitted without judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him against the expenses which such officer or director has actually and reasonably incurred.
Section 145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the
II-1
corporation as a director, officer, employee or agent of another corporation or enterprise, against any liability asserted against him and incurred by him in such capacity, or arising out of his status as such, whether or not the corporation would otherwise have the power to indemnify him under Section 145.
Our bylaws will provide that we will indemnify our directors and officers to the fullest extent authorized by the DGCL and must also pay expenses incurred in defending any such proceeding in advance of its final disposition upon delivery of an undertaking by or on behalf of an indemnified person to repay all amounts so advanced if it should be determined ultimately that such person is not entitled to be indemnified under this section or otherwise.
Upon completion of this offering, we intend to enter into indemnification agreements with each of our executive officers and directors. The indemnification agreements will provide the executive officers and directors with contractual rights to indemnification, expense advancement and reimbursement, to the fullest extent permitted under the DGCL.
The indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, provision of our certificate of incorporation or bylaws, agreement, vote of shareholders or disinterested directors or otherwise.
We will maintain standard policies of insurance that provide coverage (1) to our directors and officers against loss arising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments that we may make to such directors and officers. The proposed form of Underwriting Agreement to be filed as Exhibit 1.1 to this Registration Statement provides for indemnification of our directors and officers by the underwriters party thereto against certain liabilities arising under the Securities Act of 1933 (the “Securities Act”) or otherwise.
Item 15. Recent Sales of Unregistered Securities
Set forth below is information regarding securities sold by us within the past three years that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such securities and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
Since January 1, 2017, we have made sales of the following unregistered securities:
| • | On August 25, 2020, Maravai LifeSciences Holdings, Inc. issued 1,000 shares of its Class A common stock to Maravai LifeSciences Holdings, LLC for $10.00. The issuance of such shares of Class A common stock was not registered under the Securities Act because the shares were offered and sold in a transaction exempt from registration under Section 4(a)(2) of the Securities Act. |
The offers and sales of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act, as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the above securities represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof. Appropriate legends were placed upon any stock certificates issued in these transactions. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
II-2
Item 16. Exhibits and Financial Statement Schedules
(i) Exhibits
II-3
| * | Indicates previously filed. |
| + | Indicates a management contract or compensatory plan or agreement. |
| § | Exhibits and schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K and will be provided on a supplemental basis to the Securities and Exchange Commission upon request. |
| ‡ | Certain portions of this document that constitute confidential information have been redacted in accordance with Regulation S-K, Item 601(b)(10). |
II-4
| (ii) | Financial statement schedules |
No financial statement schedules are provided because the information called for is not applicable or is shown in the financial statements or notes.
Item 17. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in the form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective; and |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at the time shall be deemed to be the initial bona fide offering thereof. |
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on November 13, 2020.
| Maravai LifeSciences Holdings, Inc. | ||
| By: | /s/ Carl Hull | |
| Name: | Carl Hull | |
| Title: | Chief Executive Officer | |
***
POWER OF ATTORNEY
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Carl Hull Carl Hull |
Chief Executive Officer and Director (Principal Executive Officer) |
November 13, 2020 | ||
| /s/ Kevin Herde Kevin Herde |
Chief Financial Officer (Principal Financial and Accounting Officer) | November 13, 2020 | ||
II-6