
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
As submitted confidentially to the Securities and Exchange Commission on March 24, 2021 pursuant to the Jumpstart Our Business Startups Act of 2012. This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Maravai LifeSciences Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 8731 | 85-2786970 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
10770 Wateridge Circle Suite 200
San Diego, California 92121
Telephone: (858) 546-0004
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Carl W. Hull
Chief Executive Officer
10770 Wateridge Circle Suite 200
San Diego, California 92121
Telephone: (858) 546-0004
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies of all communications, including communications sent to agent for service, should be sent to:
| Robert M. Hayward, P.C. Michael P. Keeley Kirkland & Ellis LLP |
Alan F. Denenberg Davis Polk & Wardwell LLP 1600 El Camino Real Menlo Park, California 94025 (650) 752-2000 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☐
If this Form is filed to registered additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
|
| ||||||||
| Title of Each Class of Securities to be Registered |
Amount to be Registered(1) |
Proposed Maximum Offering Price Per Share(2) |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee | ||||
| Class A Common Stock, par value $0.01 per share |
||||||||
|
| ||||||||
|
| ||||||||
| (1) | Includes the aggregate offering price of shares of common stock subject to the underwriters’ option to purchase additional shares from the selling stockholders. |
| (2) | Estimated solely for purposes of computing the amount of the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended, based on the average high and low sales price of the registrant’s common stock on , 2021, as reported by the The Nasdaq Global Select Market. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
The information in this prospectus is not complete and may be changed. The selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. The prospectus is not an offer to sell these securities nor a solicitation of an offer to buy these securities in any jurisdiction where the offer and sale is not permitted.
PRELIMINARY PROSPECTUS (Subject to Completion)
Issued , 2021
Shares

Class A Common Stock
The selling stockholders named in this prospectus are offering shares of our Class A common stock. We are not selling any shares under this prospectus and we will not receive any proceeds from the sale of shares by the selling stockholders.
Our Class A common stock is listed on The Nasdaq Global Select Market, or the NASDAQ, under the symbol “MRVI”. On , 2021, the last reported sales price of our Class A common stock on The Nasdaq Global Select Market was $ per share.
Immediately after this offering, funds controlled by our principal stockholder, GTCR, LLC (“GTCR”), will control approximately % of the combined voting power of our outstanding shares of Class A common stock and Class B common stock. As a result, we will continue to be a “controlled company” within the meaning of the corporate governance standards of The Nasdaq Stock Market. See “Management—Corporate Governance—Controlled Company Status.”
Maravai LifeSciences Holdings, Inc. is an “emerging growth company” as the term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, has elected to comply with certain reduced public company reporting requirements for this prospectus.
Investing in our Class A common stock involves risks. See “Risk Factors” beginning on page 10 to read about factors you should consider before buying shares of our Class A common stock.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
| Per share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds, before expenses, to the selling stockholders |
$ | $ | ||||||
| (1) | See “Underwriters” for additional information regarding underwriting compensation. |
The underwriters have the option to purchase up to an additional shares of our Class A common stock from the selling stockholders at the public offering price less the underwriting discounts and commissions for a period of 30 days after the date of this prospectus. We will not receive any proceeds from the sale of additional shares of Class A Common Stock by the selling stockholders.
The underwriters expect to deliver shares of Class A common stock against payment in New York, New York on or about , 2021.
| MORGAN STANLEY |
Prospectus dated , 2021
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Neither we, the selling stockholders, nor any of the underwriters have authorized anyone to provide any information or make any representations other than those contained in or incorporated by reference in this prospectus or in any free writing prospectus filed with the Securities and Exchange Commission (the “SEC”). Neither we, the selling stockholders, nor the underwriters take any responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. The selling stockholders are offering to sell, and seeking offers to buy, shares of Class A common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus or the date of the applicable document incorporated by reference, regardless of the time of delivery of this prospectus or of any sale of the common stock. Our business, financial condition, results of operations, and prospects may have changed since such dates. If any statement in one of these documents is inconsistent with a statement in another document having a later date (for example, a document incorporated by reference into this prospectus) the statement in the document having the later date modifies or supersedes the earlier statement.
For investors outside of the United States, neither we, the selling stockholders, nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about, and to observe any restrictions relating to, this offering and the distribution of this prospectus outside of the United States.
i
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
BASIS OF PRESENTATION
In connection with our initial public offering (“IPO”) in November 2020, we effected certain organizational transactions. Unless otherwise stated or the context otherwise requires, all information in this prospectus reflects the consummation of the organizational transactions, including the IPO, which we refer to collectively as the “Organizational Transactions.” See “Prospectus Summary—Ownership and Organizational Structure” for a description of the Organizational Transactions and a diagram depicting our structure after giving effect to the Organizational Transactions.
Unless we state otherwise or the context otherwise requires, the terms “we,” “us,” “our,” “our business,” “the Company” and “Maravai” refer to and similar references refer: (1) on or following the consummation of the Organizational Transactions, including the IPO, to Maravai LifeSciences Holdings, Inc. and its consolidated subsidiaries, including Topco LLC, and (2) prior to the consummation of the Organizational Transactions, including the IPO, to Topco LLC and its consolidated subsidiaries. The term “GTCR” or “our Principal Stockholder” refers to GTCR, LLC, our principal stockholder, and the term “Topco LLC” refers to Maravai Topco Holdings, LLC.
We are a holding company and the sole managing member of Topco LLC and our sole asset is LLC Units of Topco LLC. Maravai LifeSciences Holdings, Inc. operates and controls all of the business and affairs and consolidates the financial results of Topco LLC. Topco LLC is the predecessor of Maravai LifeSciences Holdings, Inc. for financial reporting purposes. The Organizational Transactions are considered transactions between entities under common control. As a result, the consolidated financial statements for periods prior to the IPO and the Organizational Transactions have been adjusted to combine the previously separate entities for presentation purposes.
Unless stated otherwise, all information in this prospectus assumes no exercise by the underwriters of their option to purchase additional shares.
MARKET AND INDUSTRY DATA
Unless otherwise indicated, information included in this prospectus or incorporated by reference herein concerning economic conditions, our industry, our markets and our competitive position is based on a variety of sources, including information from independent industry analysts and publications, as well as our own estimates and research.
Our estimates are derived from publicly available information released by third-party sources, as well as data from our internal research, and are based on such data and our knowledge of our industry, which we believe to be reasonable. We have not had this information verified by any independent sources. The independent industry publications used in this prospectus or incorporated by reference in this prospectus were not prepared on our behalf. While we are not aware of any misstatements regarding any information presented in this prospectus or incorporated by reference herein, forecasts, assumptions, expectations, beliefs, estimates and projects involve risk and uncertainties and are subject to change based on various factors, including those described under the headings “Forward-Looking Statements” and “Risk Factors” in this prospectus and in our Annual Report on Form 10-K for the year ended December 31, 2020, which is incorporated by reference
TRADEMARKS AND TRADENAMES
This prospectus includes our trademarks and service marks, “Maravai LifeSciences,” “TriLink BioTechnologies,” “Glen Research,” “Cygnus Technologies,” “Vector Laboratories,” “CleanCap®,” and “MockV™,” which are protected under applicable intellectual property laws and are the property of Maravai LifeSciences Holdings, Inc. or its subsidiaries. This prospectus also contains trademarks, service marks, trade names and copyrights of other companies which are the property of their respective owners. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names.
ii
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
This summary highlights information contained elsewhere in this prospectus and in the documents incorporated by reference into this prospectus. This summary does not contain all of the information that you should consider before investing in our Class A common stock. For a more complete understanding of us and this offering, you should read and carefully consider the entire prospectus and the documents incorporated by reference herein, including the more detailed information set forth under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our consolidated financial statements and the related notes. Some of the statements in this prospectus and in the documents incorporated by reference herein are forward-looking statements. See “Forward-Looking Statements.” Unless otherwise stated, this prospectus assumes no exercise of the underwriters’ option to purchase additional shares of Class A common stock.
OVERVIEW
We are a leading life sciences company providing critical products to enable the development of drug therapies, diagnostics, novel vaccines and support research on human diseases. Our more than 5,200 customers as of December 31, 2020 include the top 20 global biopharmaceutical companies ranked by research and development expenditures according to industry consultants, and many other emerging biopharmaceutical and life sciences research companies, as well as leading academic research institutions and in vitro diagnostics companies. Our products address the key phases of biopharmaceutical development and include complex nucleic acids for diagnostic and therapeutic applications, antibody-based products to detect impurities during the production of biopharmaceutical products, and products to detect the expression of proteins in tissues of various species.
Our businesses principally address high growth market segments in biopharmaceutical development that are growing at a weighted average blended rate of 20% per annum. In particular, the field of cell and gene therapy has emerged as one of the fastest growing treatment modalities to address a host of human conditions. There are more than 400 cell and gene therapies in development or launched and sales in this category are expected to grow more than tenfold by 2024, according to industry consultants and management estimates. Our portfolio offers key products for each stage of the cell and gene therapy development lifecycle. For example, our mRNA products are used in drug development to assist in the production of immune-activating antigens; our CleanCap® technology is used to stabilize mRNA and streamlines mRNA manufacturing; and we expect our upcoming plasmid DNA products will be used as templates for the production of our RNA products. We also provide biologics safety testing technology used to ensure the safety of the biological drug manufacturing process and drug products.
mRNA is at the core of our capabilities. We developed our expertise in mRNA with a belief in its potential as a therapeutic modality. The first clinical trial for an mRNA therapeutic agent occurred in 2016. More than 30 clinical trials have occurred since then, principally focused on vaccines against viruses and cancer vaccines. With the COVID-19 pandemic, mRNA has shown its potential for more rapid vaccine design and manufacture when compared to traditional techniques involving culturing inactivated virus to elicit an immune response.
For a description of our business, financial condition, results of operations and other important information regarding us, see our filings with the SEC incorporated by reference in this prospectus. For instructions on how to find copies of the filings incorporated by reference in this prospectus, see “Where You Can Find More Information.”
1
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
OUR PRINCIPAL STOCKHOLDER
We have a valuable relationship with our principal stockholder, GTCR. Founded in 1980, GTCR is a leading growth-oriented private equity firm focused on investing in growth companies in the Healthcare, Financial Services & Technology, Technology, Media & Telecommunications and Growth Business Services industries. The Chicago-based firm pioneered The Leaders Strategy™—finding and partnering with management leaders in core domains to identify, acquire and build market-leading companies through transformational acquisitions and organic growth. Maravai is an example of the Leaders Strategy™, whereby GTCR partnered with Carl Hull and Eric Tardif to build and grow a leading life sciences platform. Since its inception, GTCR has invested more than $20.0 billion in over 250 companies.
GENERAL CORPORATE INFORMATION
We completed our IPO on November 19, 2020, and our Class A common stock commenced trading on The Nasdaq Global Select Market. Our principal executive offices are located at 10770 Wateridge Circle Suite 200, San Diego, California, 92121. Our telephone number is (858) 546-0004. Our website address is www.maravai.com. The information contained on, or that can be accessed through, our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our Class A common stock. We are a holding company and all of our business operations are conducted through, and substantially all of our assets are held by, our subsidiaries.
IMPLICATIONS OF BEING AN EMERGING GROWTH COMPANY
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). We will remain an emerging growth company until the earlier of (1) December 31, 2025, (2) the last day of the fiscal year in which we have total annual gross revenue of at least $1.07 billion, (3) the date on which we are deemed to be a large accelerated filer (this means the market value of common that is held by non-affiliates exceeds $700.0 million as of the end of the second quarter of that fiscal year), or (4) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies until they no longer qualify as an emerging growth company. These provisions include, but are not limited to:
| • | not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (the “Sarbanes-Oxley Act”); |
| • | only required to present two years of audited financial statements, plus unaudited condensed financial statements for any interim period, and related management’s discussion and analysis of financial condition and results of operations; |
| • | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
| • | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
2
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
We have elected to take advantage of certain of the reduced disclosure obligations regarding financial statements and executive compensation in this prospectus and expect to elect to take advantage of other reduced burdens in future filings until we no longer qualify as an emerging growth company. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
Under the JOBS Act emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to avail ourselves of the extended transition period for complying with new or revised financial accounting standards. As a result of the accounting standards election, we will not be subject to the same implementation timing for new or revised accounting standards as other public companies that are not emerging growth companies, which may make comparison of our financials to those of other public companies more difficult.
OWNERSHIP AND ORGANIZATIONAL STRUCTURE
Maravai LifeSciences Holdings, Inc. is a Delaware corporation that was formed to serve as a holding company and holds an interest in Topco LLC. Maravai LifeSciences Holdings, Inc. has not engaged in any business or other activities other than in connection with its formation, the Organizational Transactions, the IPO and this offering.
Immediately prior to the consummation of our IPO, we and the other direct and indirect unitholders of Topco LLC, effected a series of transactions that resulted in (i) Maravai LifeSciences Holdings, Inc. becoming the ultimate parent company of Topco LLC and (ii) the then-current unitholders in Topco LLC exchanging their interests in Topco LLC for Class A common stock of Maravai LifeSciences Holdings, Inc. We refer to these transactions as the “Organizational Transactions.”
| • | We amended and restated Topco LLC’s existing operating agreement (the “LLC Operating Agreement”) to, among other things, (i) modify Topco LLC’s capital structure by replacing the membership interests currently held by Topco LLC’s existing owners (beneficially owned through Maravai Life Sciences Holdings, LLC (“MLSH 1”)) with a new class of LLC Units held initially by MLSH 1 and (ii) appoint Maravai LifeSciences Holdings, Inc. as the sole managing member of Topco LLC. |
| • | Certain of the entities (the “Blocker Entities”) through which GTCR and other existing members of MLSH 1 held their ownership interests in MLSH 1 formed Maravai Life Sciences Holdings 2, LLC (“MLSH 2”) and engaged in a series of transactions (the “Blocker Mergers”) that resulted in each of the Blocker Entities merging with and into Maravai LifeSciences Holdings, Inc., with Maravai LifeSciences Holdings, Inc. remaining as the surviving corporation. As a result of such transactions, (i) the former unitholders of the Blocker Entities became members of MLSH 2 and (ii) MLSH 2 exchanged all of the equity interests in the Blocker Entities for (x) shares of Class A common stock and (y) the right to receive payments pursuant to the Tax Receivable Agreement. |
| • | We entered into an exchange agreement (the “Exchange Agreement”) with MLSH 1 pursuant to which MLSH 1 is entitled to exchange LLC Units, together with an equal number of shares of Class B common stock, for shares of Class A common stock on a one-for-one basis or, at our election, for cash, from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). |
| • | We entered into a tax receivable agreement (the “Tax Receivable Agreement”) with MLSH 1 and MLSH 2 that provides for the payment by Maravai LifeSciences Holdings, Inc. to MLSH 1 and MLSH 2, collectively, of 85% of the amount of cash savings, if any, in U.S. federal, state and local income |
3
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| taxes (computed using simplifying assumptions to address the impact of state and local taxes) we actually realize (or, under certain circumstances are deemed to realize in the case of an early termination payment by us, a change in control or a material breach by us of our obligations under the Tax Receivable Agreement, as discussed below) as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to the IPO and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we are required to make under the Tax Receivable Agreement. |
The diagram below depicts our current organizational structure. This diagram is provided for illustrative purposes only and does not purport to represent all legal entities owned or controlled by us, or owning a beneficial interest in us.
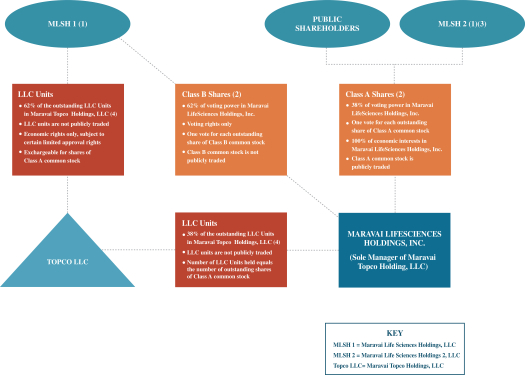
| (1) | Upon completion of this offering, GTCR will continue to control the voting power in Maravai LifeSciences Holdings, Inc. as follows: (i) approximately % through its control of MLSH 1, or % if the underwriters exercise in full their option to purchase additional shares, and (ii) approximately % through its control of MLSH 2, or % if the underwriters exercise in full their option to purchase additional shares. See “Principal and Selling Stockholders” for additional information about MLSH 1 and MLSH 2. |
| (2) | Shares of Class A common stock and Class B common stock vote as a single class. Each outstanding share of Class A common stock and Class B Common stock is entitled to one vote on all matters to be voted on by stockholders generally. The Class B common stock does not have any right to receive dividends or distributions upon the liquidation or winding up of Maravai LifeSciences Holdings, Inc. In accordance with the Exchange Agreement entered into in connection with the Organizational Transactions, MLSH 1 is entitled to exchange LLC Units, together with an equal number of shares of Class B common stock, for |
4
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| shares of Class A common stock determined in accordance with the Exchange Agreement or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). |
| (3) | Upon completion of the IPO, we awarded options to purchase an aggregate of 1,522,100 shares of Class A common stock with an exercise price set at the IPO price of $27.00 issued pursuant to the 2020 Omnibus Incentive Plan (the “2020 Plan”). |
| (4) | Upon completion of this offering, (i) the holders of Class A common stock will have % of the voting power in Maravai LifeSciences Holdings, Inc., (ii) MLSH 1, through ownership of the Class B common stock, will have % of the voting power of Maravai LifeSciences Holdings, Inc., (iii) MLSH 1 will own % of the outstanding LLC Units in Topco LLC and (iv) Maravai LifeSciences Holdings, Inc. will own % of the outstanding LLC Units in Topco LLC. |
Our corporate structure, as described above, is commonly referred to as an “Up-C” structure, which is commonly used by partnerships and limited liability companies when they undertake an initial public offering of their business. Our Up-C structure together with the Tax Receivable Agreement allows the existing owners of Topco LLC to continue to realize tax benefits associated with owning interests in an entity that is treated as a partnership, or “passthrough” entity, for income tax purposes. One of these benefits is that taxable income of the Topco LLC that is allocated to such owners will be taxed on a flow-through basis and therefore will not be subject to corporate taxes at the entity level. Additionally, because the LLC Units that the existing owners continue to hold are exchangeable for shares of our Class A common stock or, at our option, for cash, from Topco LLC, the Up-C structure also provides the existing owners of Topco LLC potential liquidity that holders of non-publicly traded limited liability companies are not typically afforded. See “Description of Capital Stock.”
MLSH 1 holds a number of shares of our Class B common stock equal to the number of LLC Units it owns. Holders of our Class A common stock and Class B common stock are each entitled to one vote per share on all matters on which stockholders are entitled to vote.
Maravai LifeSciences Holdings, Inc. holds LLC Units, and therefore receive benefits on account of its ownership in an entity treated as a partnership, or “passthrough” entity, for income tax purposes. As Maravai LifeSciences Holdings, Inc. purchases LLC Units from MLSH 1 under the mechanism described above, it will obtain a step-up in tax basis in its share of the assets of Topco LLC and its flow-through subsidiaries. This step-up in tax basis will provide Maravai LifeSciences Holdings, Inc. with certain tax benefits, such as future depreciation and amortization deductions that can reduce the taxable income allocable to Maravai LifeSciences Holdings, Inc. Pursuant to the Tax Receivable Agreement, Maravai LifeSciences Holdings, Inc. agreed to pay MLSH 1 and MLSH 2, collectively, 85% of the value of these tax benefits; however, the remaining 15% of such benefits is available to Maravai LifeSciences Holdings, Inc. Due to the uncertainty of various factors, we cannot precisely quantify the likely tax benefits we will realize as a result of LLC Unit exchanges and the resulting amounts we are likely to pay out to LLC Unitholders pursuant to the Tax Receivable Agreement; however, we estimate that such payments may be substantial.
Generally, Maravai LifeSciences Holdings, Inc. will receive a pro rata share of any distributions (including tax distributions) made by Topco LLC to its members. Tax distributions will be calculated without regard to any applicable basis adjustment under Section 743(b) of the Internal Revenue Code (the “Code”) and will be based upon an assumed tax rate, which, under certain circumstances, may cause Topco LLC to make tax distributions that, in the aggregate, exceed the amount of taxes that Topco LLC would have paid if it were a similarly situated corporate taxpayer. Funds used by Topco LLC to satisfy its tax distribution obligations will not be available for reinvestment in our business.
5
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
THE OFFERING
| Class A common stock offered by the selling |
shares. | |
| Option to purchase additional shares of Class A common stock from the selling stockholders |
The underwriters have the option to purchase up to an additional shares from the selling stockholders, at the public offering price, less the underwriting discount, within 30 days of the date of this prospectus. | |
| Class A common stock to be outstanding immediately after this offering |
shares. If all outstanding LLC Units (together with the same number of shares of our Class B common stock) held by MLSH 1 were exchanged for newly-issued shares of Class A common stock on a one-for-one basis, shares of Class A common stock would be outstanding | |
| Class B common stock to be outstanding immediately after this offering |
shares. | |
| Use of proceeds |
The selling stockholders will receive all of the net proceeds from this offering and we will not receive any proceeds from the sale of shares of Class A common stock in this offering. See “Use of Proceeds”. | |
| Risk factors |
Investing in our Class A common stock involves a high degree of risk. See “Risk Factors” elsewhere in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our Class A common stock. | |
| Voting power held by holders of Class A common stock immediately after this offering |
% | |
| Voting power held by holders of Class B common stock immediately after this offering |
% | |
| Symbol for trading on The Nasdaq Global Select |
“MRVI.” | |
The number of shares of Class A common stock to be outstanding following this offering is based on shares of Class A common stock outstanding as of December 31, 2020, and excludes:
| • | shares of Class A common stock that may be issuable upon exercise of redemption and exchange rights held by MLSH 1, as of December 31, 2020; |
| • | shares of Class A common stock, plus future increases, reserved for issuance under our 2020 Employee Stock Purchase Plan (the “ESPP”), as of December 31, 2020; and |
| • | shares of Class A common stock reserved for future issuance under the 2020 Plan, including (i) options to purchase shares of Class A common stock issued to certain employees upon completion of the IPO, with an exercise price set at the IPO price of $27.00 per share and that vest in |
6
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| accordance with the schedule described in “Executive and Director Compensation” and (ii) restricted stock units (“RSUs”) that may be settled for an equal number of shares of Class A common stock that were issue to our six independent directors upon completion of the IPO and that vest annually over three years, each as of December 31, 2020. |
Unless otherwise indicated, all information in this prospectus assumes:
| • | no exercise of outstanding options or issuance of shares of Class A common stock upon vesting and settlement RSUs after December 31, 2020; and |
| • | no exercise by the underwriters of their option to purchase up to additional shares of Class A common stock from any of the selling stockholders. |
7
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
SUMMARY HISTORICAL FINANCIAL AND OTHER DATA
The following tables summarize our consolidated financial data. The summary consolidated statement of operations data for the years ended December 31, 2020, 2019 and 2018 and the summary consolidated balance sheet data as of December 31, 2020 and 2019 are derived from our audited consolidated financial statements that are incorporated by reference into this prospectus.
Our historical results are not necessarily indicative of the results that may be expected in the future. You should read the summary historical financial data below in conjunction with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and related notes appearing in our Annual Report on Form 10-K for the year ended December 31, 2020, which is incorporated by reference into this prospectus.
| Year Ended December 31, |
||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| (in thousands, except share and unit amounts and per share and per unit amounts) |
||||||||||||
| Revenue |
$ | 284,098 | $ | 143,140 | $ | 123,833 | ||||||
| Operating expenses |
||||||||||||
| Cost of revenue |
79,649 | 66,849 | 60,765 | |||||||||
| Research and development |
9,304 | 3,627 | 4,499 | |||||||||
| Selling, general and administrative |
94,245 | 48,354 | 41,194 | |||||||||
| Change in estimated fair value of contingent consideration |
— | 322 | 939 | |||||||||
| Gain on sale and leaseback transaction |
(19,002 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
164,196 | 119,152 | 107,397 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income from operations |
119,902 | 23,988 | 16,436 | |||||||||
| Other income (expense) |
||||||||||||
| Interest expense |
(30,740 | ) | (29,959 | ) | (27,399 | ) | ||||||
| Loss on extinguishment of debt |
(7,592 | ) | — | (5,622 | ) | |||||||
| Other income |
126 | 118 | 87 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income (loss) before income taxes |
81,696 | (5,853 | ) | (16,498 | ) | |||||||
| Income tax expense (benefit) |
2,880 | (652 | ) | 417 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) |
78,816 | (5,201 | ) | (16,915 | ) | |||||||
| Net (loss) attributable to noncontrolling interests |
(10,156 | ) | (731 | ) | (12,443 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) attributable to Maravai LifeSciences Holdings, Inc. |
$ | 88,972 | $ | (4,470 | ) | $ | (4,472 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) per Class A common share/unit attributable to Maravai LifeSciences Holdings, Inc.: |
||||||||||||
| Basic |
$ | 7.43 | $ | (0.03 | ) | $ | (0.07 | ) | ||||
| Diluted |
$ | 2.36 | $ | (0.03 | ) | $ | (0.07 | ) | ||||
| Weighted average number of Class A common shares/units outstanding: |
||||||||||||
| Basic |
10,351,137 | 253,916,941 | 253,916,941 | |||||||||
| Diluted |
28,907,979 | 253,916,941 | 253,916,941 | |||||||||
8
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| As of December 31, 2020 |
As of December 31, 2019 |
|||||||
| (in thousands) | ||||||||
| Consolidated Balance Sheet Data: |
||||||||
| Cash |
$ | 236,184 | $ | 24,700 | ||||
| Working capital(1) |
200,820 | 30,990 | ||||||
| Total assets |
1,270,691 | 577,796 | ||||||
| Long-term debt, less current portion |
528,614 | 334,783 | ||||||
| Total liabilities |
1,115,945 | 433,169 | ||||||
| Total member’s/shareholders’ equity |
154,746 | 144,627 | ||||||
| (1) | We define working capital as current assets less current liabilities. |
9
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Investing in our Class A common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, as well as the risks and uncertainties set forth under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2020, which is incorporated by referenced herein, together with all of the other information included or incorporated by reference in this prospectus, including our consolidated financial statements and the related notes thereto, before making a decision to invest in our Class A common stock. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. If any of the following risks occur, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that event, the price of our Class A common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Class A Common Stock and This Offering
GTCR controls us, and its interests may conflict with ours or yours in the future.
Immediately following this offering, investment entities affiliated with GTCR will control approximately % of the voting power of our outstanding common stock, or % if the underwriters exercise in full their option to purchase additional shares, which means that, based on its percentage voting power controlled after the offering, GTCR will continue to control the vote of all matters submitted to a vote of our stockholders. This control enables GTCR to control the election of the members of our board of directors (the “Board”) and all other corporate decisions. Even when GTCR ceases to control a majority of the total voting power, for so long as GTCR continues to own a significant percentage of our common stock, GTCR will still be able to significantly influence the composition of our Board and the approval of actions requiring stockholder approval. Accordingly, for such period of time, GTCR will have significant influence with respect to our management, business plans and policies, including the appointment and removal of our officers, decisions on whether to raise future capital and amending our charter and bylaws, which govern the rights attached to our common stock. In particular, for so long as GTCR continues to own a significant percentage of our common stock, GTCR will be able to cause or prevent a change of control of us or a change in the composition of our Board and could preclude any unsolicited acquisition of us. The concentration of ownership could deprive you of an opportunity to receive a premium for your shares of Class A common stock as part of a sale of us and ultimately might affect the market price of our Class A common stock.
In addition, in connection with our IPO, we entered into a Director Nomination Agreement with GTCR that provides GTCR the right to nominate to the Board a number of designees equal to at least: (i) 100% of the total number of directors comprising the Board, so long as GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 40% of the total amount of shares of Class A common stock and Class B common stock it beneficially owned as of the date of the IPO, (ii) 40% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 30% but less than 40% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO, (iii) 30% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 20% but less than 30% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO, (iv) 20% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 10% but less than 20% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO and (v) one director, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 5% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO. The Director Nomination Agreement provides that GTCR may assign such right to a GTCR affiliate. The Director Nomination Agreement prohibits us from increasing or decreasing the size of our Board without the prior written consent of GTCR. See “Certain Relationships and Related Party Transactions—Director Nomination Agreement” for more details with respect to the Director Nomination Agreement.
10
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
GTCR and its affiliates engage in a broad spectrum of activities, including investments in our industry generally. In the ordinary course of their business activities, GTCR and its affiliates may engage in activities where their interests conflict with our interests or those of our other stockholders, such as investing in or advising businesses that directly or indirectly compete with certain portions of our business or are suppliers or customers of ours. Our certificate of incorporation provides that none of GTCR, any of its affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his or her director and officer capacities) or its affiliates has any duty to refrain from engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. GTCR also may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. In addition, GTCR may have an interest in pursuing acquisitions, divestitures and other transactions that, in its judgment, could enhance its investment, even though such transactions might involve risks to you or may not prove beneficial.
We are a “controlled company” within the meaning of the rules of NASDAQ and, as a result, we qualify for, and rely on, exemptions from certain corporate governance requirements. You will not have the same protections as those afforded to stockholders of companies that are subject to such governance requirements.
After completion of this offering, GTCR will continue to control a majority of the voting power of our outstanding common stock. As a result, we continue to be a “controlled company” within the meaning of the corporate governance standards of NASDAQ. Under these rules, a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including:
| • | the requirement that a majority of our Board consist of independent directors; |
| • | the requirement that we have a nominating and corporate governance committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; |
| • | the requirement that we have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| • | the requirement for an annual performance evaluation of the nominating and corporate governance and compensation committees. |
Following this offering, we will continue to utilize these exceptions. As a result, we may not have a majority of independent directors on our Board, our compensation and nominating and corporate governance committees may not consist entirely of independent directors and our compensation and nominating and corporate governance committees may not be subject to annual performance evaluations. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of NASDAQ.
For so long as we are an “emerging growth company”, we will not be required to comply with certain public company reporting requirements, which could make our Class A common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we are eligible for certain exemptions from various public company reporting requirements. These exemptions include, but are not limited to, (i) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (ii) reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements, (iii) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved and (iv) not being required to provide five
11
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
years of Selected Consolidated Financial Data in this prospectus. We could be an emerging growth company for up to five years after the first sale of our Class A common stock in our IPO, which fifth anniversary will occur in 2025. However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenue exceeds $1.07 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we would cease to be an emerging growth company prior to the end of such five-year period. We have made certain elections with regard to the reduced disclosure obligations regarding executive compensation in this prospectus and may elect to take advantage of other reduced disclosure obligations in future filings. As a result, the information that we provide to holders of our Class A common stock may be different than you might receive from other public reporting companies in which you hold equity interests. We cannot predict if investors will find our Class A common stock less attractive as a result of reliance on these exemptions. If some investors find our Class A common stock less attractive as a result of any choice we make to reduce disclosure, there may be a less active trading market for our Class A common stock and the market price for our Class A common stock may be more volatile.
The JOBS Act also permits an emerging growth company like us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We previously elected to take advantage of this extended transition period for complying with new or revised accounting standards provided for by the JOBS Act. We will therefore comply with new or revised accounting standards when they apply to private companies. As a result, our financial statements may not be comparable with companies that comply with public company effective dates for accounting standards.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business, particularly after we are no longer an “emerging growth company.”
As a public company, we incur legal, accounting and other expenses that we did not incur before our IPO. We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and the Sarbanes-Oxley Act, the listing requirements of NASDAQ and other applicable securities rules and regulations. Compliance with these rules and regulations will continue to increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and continue to increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.” The Exchange Act requires that we file annual, quarterly and current reports with respect to our business, financial condition, results of operations, cash flows and prospects. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective internal controls and procedures for financial reporting. Furthermore, the need to establish the corporate infrastructure demanded of a public company may divert our management’s attention from implementing our growth strategy, which could prevent us from improving our business, financial condition, results of operations, cash flows and prospects. We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a public company. However, the measures we take may not be sufficient to satisfy our obligations as a public company. In addition, these rules and regulations increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, these rules and regulations make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. These additional obligations could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance
12
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
practices. We intend to continue to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of our management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and there could be a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Provisions of our corporate governance documents could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our current management, even if beneficial to our stockholders.
Our certificate of incorporation and bylaws and the Delaware General Corporation Law (the “DGCL”) contain provisions that could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our stockholders. Among other things:
| • | these provisions allow us to authorize the issuance of undesignated preferred stock, the terms of which may be established and the shares of which may be issued without stockholder approval, and which may include supermajority voting, special approval, dividend, or other rights or preferences superior to the rights of stockholders; |
| • | these provisions provide for a classified board of directors with staggered three-year terms; |
| • | these provisions provide that, at any time when GTCR controls, in the aggregate, less than 40% of the outstanding shares of our Class A common stock, directors may only be removed for cause, and only by the affirmative vote of holders of at least 662/3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class; |
| • | these provisions prohibit stockholder action by written consent from and after the date on which GTCR controls, in the aggregate, less than 35% in voting power of our stock entitled to vote generally in the election of directors; |
| • | these provisions provide that for as long as GTCR controls, in the aggregate, at least 50% in voting power of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our stockholders will require the affirmative vote of a majority in voting power of the outstanding shares of our capital stock and at any time when GTCR controls, in the aggregate, less than 50% in voting power of all outstanding shares of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our stockholders will require the affirmative vote of the holders of at least 662/3% in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class; and |
| • | these provisions establish advance notice requirements for nominations for elections to our Board or for proposing matters that can be acted upon by stockholders at stockholder meetings; provided, however, at any time when GTCR controls, in the aggregate, at least 10% in voting power of our stock entitled to vote generally in the election of directors, such advance notice procedure will not apply to GTCR. |
We opted out of Section 203 of the DGCL, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder. However, our certificate of incorporation contains a provision that provides us with protections similar to Section 203, and prevents us from engaging in a business combination with a person (excluding GTCR and any of its direct or indirect transferees and any group as to which such persons are a party) who acquires at least 85% of our common stock for a period of three years from the date such person acquired such common stock, unless board or stockholder approval is obtained prior to the acquisition. See “Description of Capital Stock—Anti-Takeover Provisions.” These
13
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
provisions could discourage, delay or prevent a transaction involving a change in control of our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing and cause us to take other corporate actions you desire, including actions that you may deem advantageous, or negatively affect the trading price of our Class A common stock. In addition, because our Board is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team.
These and other provisions in our certificate of incorporation, bylaws and Delaware law could make it more difficult for stockholders or potential acquirers to obtain control of our Board or initiate actions that are opposed by our then-current Board, including actions to delay or impede a merger, tender offer or proxy contest involving our company. The existence of these provisions could negatively affect the price of our Class A common stock and limit opportunities for you to realize value in a corporate transaction.
For information regarding these and other provisions, see “Description of Capital Stock.”
Our certificate of incorporation designates the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation that may be initiated by our stockholders and the federal district courts of the United States as the exclusive forum for litigation arising under the Securities Act, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us.
Pursuant to our certificate of incorporation, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any claims in state court for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, (3) any action asserting a claim against us arising pursuant to any provision of the DGCL, our certificate of incorporation or our bylaws or (4) any other action asserting a claim against us that is governed by the internal affairs doctrine; provided that for the avoidance of doubt, the forum selection provision that identifies the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation, including any “derivative action,” will not apply to suits to enforce a duty or liability created by the Securities Act, the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Our certificate of incorporation further provides that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock is deemed to have notice of and consented to the provisions of our certificate of incorporation described above. See “Description of Capital Stock—Forum Selection.” The forum selection provisions in our certificate of incorporation may have the effect of discouraging lawsuits against us or our directors and officers and may limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us. If the enforceability of our forum selection provisions were to be challenged, we may incur additional costs associated with resolving such challenge. While we currently have no basis to expect any such challenge would be successful, if a court were to find our forum selection provisions to be inapplicable or unenforceable with respect to one or more of these specified types of actions or proceedings, we may incur additional costs associated with having to litigate in other jurisdictions, which could have an adverse effect on our business, financial condition, results of operations, cash flows and prospects and result in a diversion of the time and resources of our employees, management and board of directors.
An active, liquid trading market for our Class A common stock may not be sustained, which may limit your ability to sell your shares.
Our IPO occurred in November 2020. Therefore, there has only been a public market for our Class A common stock for a short period of time. Although our Class A common stock was listed on The Nasdaq Global Select Market under the trading symbol “MRVI,” there is a very limited trading history and an active trading market for our Class A common stock may not be sustained. The public offering price was determined by
14
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
negotiations between the selling stockholders and the underwriters and may not be indicative of market prices of our Class A common stock that prevail in the open market after the offering. A public trading market having the desirable characteristics of depth, liquidity and orderliness depends upon the existence of willing buyers and sellers at any given time, such existence being dependent upon the individual decisions of buyers and sellers over which neither we nor any market maker has control. The failure of an active and liquid trading market to continue would likely have a material adverse effect on the value of our Class A common stock. The market price of our Class A common stock may decline below the public offering price, and you may not be able to sell your shares of our Class A common stock at or above the price you paid in this offering, or at all. An inactive market may also impair our ability to raise capital to continue to fund operations by issuing additional shares of our Class A common stock or other equity or equity-linked securities and may impair our ability to acquire other companies or technologies by using any such securities as consideration.
Our operating results and stock price may be volatile, and the market price of our Class A common stock after this offering may drop below the price you pay.
Our quarterly operating results are likely to fluctuate in the future. In addition, securities markets worldwide have experienced, and are likely to continue to experience, significant price and volume fluctuations, including as a result of the COVID-19 pandemic. This market volatility, as well as general economic, market or political conditions, could subject the market price of our Class A common stock to wide price fluctuations regardless of our operating performance. Our operating results and the trading price of our Class A common stock may fluctuate in response to various factors, including:
| • | market conditions in our industry or the broader stock market; |
| • | actual or anticipated fluctuations in our quarterly financial and operating results; |
| • | introduction of new products or services by us or our competitors; |
| • | issuance of new or changed securities analysts’ reports or recommendations; |
| • | sales, or anticipated sales, of large blocks of our stock; |
| • | additions or departures of key personnel; |
| • | regulatory or political developments; |
| • | litigation and governmental investigations; |
| • | any coordinated trading activities or large derivative positions in our Class A common stock, for example, a “short squeeze” (a short squeeze occurs when a number of investors take a short position in a stock and have to buy the borrowed securities to close out the position at a time that other short sellers of the same security also want to close out their positions, resulting in surges stock prices, i.e., demand is greater than supply for the stock sold shorted); |
| • | changing economic conditions; |
| • | investors’ perception of us; |
| • | events beyond our control such as weather, war and health crises such as the COVID-19 pandemic; and |
| • | any default on our indebtedness. |
These and other factors, many of which are beyond our control, may cause our operating results and the market price and demand for our Class A common stock to fluctuate substantially. Fluctuations in our quarterly operating results could limit or prevent investors from readily selling their shares of Class A common stock and may otherwise negatively affect the market price and liquidity of our shares of Class A common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the company that issued the stock. If any of our stockholders
15
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management from our business, which could significantly harm our profitability and reputation.
In addition, the stock markets in general, and the markets for pharmaceutical, biopharmaceutical and biotechnology stocks in particular, have experienced extreme volatility, including as a result of the COVID-19 pandemic, that has often been unrelated to the operating performance of the issuer.
A significant portion of our total outstanding shares of Class A common stock are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our Class A common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our Class A common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares of Class A common stock intend to sell shares, could reduce the market price of our Class A common stock. After this offering, we will have outstanding shares of Class A common stock based on the number of shares outstanding as of December 31, 2020. This includes shares of Class A common stock that the selling stockholders are selling in this offering, which may be resold in the public market immediately unless purchased by one of our affiliates.
In connection with this offering, Morgan Stanley & Co. LLC, and , as representatives of the several underwriters of our IPO, have agreed to release the restrictions under the lock-up agreements that were executed in connection with our IPO with respect to up to shares of our Class A common stock to be sold in this offering that are held by the selling stockholders, which includes shares beneficially owned by our directors and executive officers or entities with which they are affiliated; provided, however, that the release of shares of our common stock held by the selling stockholders is limited to the shares actually sold in this offering. Morgan Stanley & Co. LLC, and , as representatives of the several underwriters of our IPO, may, in their sole discretion, from time to time permit our stockholders to sell additional shares and waive the contractual lock-up prior to the expiration of the lock-up agreements.
In addition, subject to certain exceptions described in the section titled “Underwriters,” our directors and executive officers and GTCR have entered into lock-up agreements with the underwriters of this offering pursuant to which they have agreed that subject to certain exceptions, they will not directly or indirectly sell or dispose of any shares of Class A common stock or any securities convertible into or exchangeable or exercisable for shares of Class A common stock for a period of 90 days after the date of this prospectus. See “Underwriters” and “Shares Eligible for Future Sale” for more information.
All of these shares will, however, be able to be resold after the expiration of the lock-up period, as well as pursuant to customary exceptions thereto or upon the waiver of the lock-up agreement by the representatives on behalf of the underwriters. We have registered shares of Class A common stock that we may issue under our equity compensation plans. Such shares can be freely sold in the public market upon issuance. As restrictions on resale end, the market price of our stock could decline if the holders of currently restricted shares of Class A common stock sell them or are perceived by the market as intending to sell them.
Because we have no current plans to pay cash dividends on our Class A common stock for the foreseeable future, you may not receive any return on investment unless you sell your Class A common stock for a price greater than that which you paid for it.
We do not anticipate paying any cash dividends on our Class A common stock for the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of our Board and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that our Board may deem relevant. In addition, our ability to pay dividends is, and
16
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
may be, limited by covenants of existing and any future outstanding indebtedness we or our subsidiaries incur, including under the Credit Agreement that we entered into on October 19, 2020 (the “New Credit Agreement”). Therefore, any return on investment in our Class A common stock is solely dependent upon the appreciation of the price of our Class A common stock on the open market, which may not occur. See “Dividend Policy” for more detail.
If securities or industry analysts do not publish research or reports about our business, if they publish unfavorable research or reports, or adversely change their recommendations regarding our Class A common stock or if our results of operations do not meet their expectations, our stock price and trading volume could decline.
The trading market for our shares is influenced by the research and reports that industry or securities analysts publish about us or our business. We do not have any control over these analysts. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline. Moreover, if one or more of the analysts who cover us downgrade our stock, or if our results of operations do not meet their expectations, our stock price could decline.
We may issue shares of preferred stock in the future, which could make it difficult for another company to acquire us or could otherwise adversely affect holders of our Class A common stock, which could depress the price of our Class A common stock.
Our certificate of incorporation authorizes us to issue one or more series of preferred stock. Our Board has the authority to determine the preferences, limitations and relative rights of the shares of preferred stock and to fix the number of shares constituting any series and the designation of such series, without any further vote or action by our stockholders. Our preferred stock could be issued with voting, liquidation, dividend and other rights superior to the rights of our Class A common stock. The potential issuance of preferred stock may delay or prevent a change in control of us, discouraging bids for our Class A common stock at a premium to the market price, and materially adversely affect the market price and the voting and other rights of the holders of our Class A common stock.
17
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
This prospectus contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties. All statements other than statements of historical fact included in this prospectus or incorporated by reference herein are forward-looking statements. Forward-looking statements give our current expectations and projections relating to our financial condition, results of operations, plans, objectives, future performance and business. You can identify forward-looking statements by the fact that they do not relate strictly to historical or current facts. These statements may include words such as “anticipate,” “estimate,” “expect,” “project,” “plan,” “intend,” “believe,” “may,” “will,” “should,” “can have,” “likely” and other words and terms of similar meaning in connection with any discussion of the timing or nature of future operating or financial performance or other events. For example, all statements we make relating to our estimated and projected costs, expenditures, cash flows, growth rates and financial results, our plans and objectives for future operations, growth or initiatives, strategies or the expected outcome or impact of pending or threatened litigation are forward-looking statements. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected, including:
| • | our history of losses, the risk that we may continue to incur losses in the future and our ability to generate sufficient revenue to achieve or maintain profitability; |
| • | the fluctuation of our operating results, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide; |
| • | our dependence on a limited number of customers for a high percentage of our revenue; |
| • | the use of certain of our products in the production of vaccines and therapies that represent relatively new and still-developing modes of treatment, which may experience unforeseen adverse events, negative clinical outcomes or increased regulatory scrutiny; |
| • | the impact of COVID-19 and any pandemic, epidemic or outbreak of infectious disease; |
| • | changes in economic conditions; |
| • | our dependence on customers’ spending on and demand for outsourced nucleic acid production, biologics safety testing and protein detection research products and services; |
| • | competition with life science, pharmaceutical and biotechnology companies who are substantially larger than we are and potentially capable of developing new approaches that could make our products, services and technologies obsolete; |
| • | the ability of our products and services to perform as expected and the reliability of the technology on which our products and services are based; |
| • | the complexity of our products and the fact that they are subject to quality control requirements; |
| • | our reliance on a limited number of suppliers or, in some cases, sole suppliers, for some of our raw materials and our inability to find replacements or immediately transition to alternative suppliers; |
| • | our dependence on a stable and adequate supply of quality raw materials from our suppliers, and the risk of adverse impacts from price increases or interruptions of such supply; |
| • | disruptions at our sites; |
| • | our ability to manufacture in specific quantities; |
| • | natural disasters, geopolitical unrest, war, terrorism, public health issues such as COVID-19 or other catastrophic events that could disrupt the supply, delivery or demand of products and services; |
| • | our ability to secure additional financing for future strategic transactions; |
| • | our reliance on third-party package delivery services and adverse impacts arising from significant disruptions of these services, damages or losses sustained during shipping or significant increases in prices; |
18
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| • | our ability to continue to hire and retain skilled personnel; |
| • | our ability to successfully identify and implement distribution arrangements and marketing alliances; |
| • | the market acceptance of our life science reagents; |
| • | the market receptivity to our new products and services upon their introduction; |
| • | our ability to implement our strategies for revenue growth; |
| • | the accuracy of our estimates of market opportunity and forecasts of market growth included in this prospectus; |
| • | product liability lawsuits; |
| • | the application of privacy laws, security laws, regulations, policies and contractual obligations related to data privacy and security; |
| • | our ability to efficiently manage our growth; |
| • | the success of any opportunistic acquisitions; |
| • | the integrity of our internal computer systems; |
| • | the impact of export and import control laws and regulations; |
| • | risks related to Brexit; |
| • | changes in political, economic or governmental regulations; |
| • | financial, operating, legal and compliance risks associated with global operations; |
| • | risks associated with our acquisitions; |
| • | impacts from foreign currency exchange rates; |
| • | the risk that our products could become subject to more onerous regulation in the future; |
| • | our ability to use net operating loss and tax credit carryforwards; |
| • | the fact that our activities are and will continue to be subject to extensive government regulation; |
| • | the risk that we may be required to record a significant charge to earnings if our goodwill or other amortizable intangible assets become impaired; |
| • | unfavorable accounting charges or effects driven by changes in accounting principles or guidance; |
| • | impacts on our financial results from our revenue recognition and other factors; |
| • | fluctuations in our effective tax rate; |
| • | environmental risks; |
| • | our ability to obtain, maintain and enforce intellectual property protection for our current and future products; |
| • | our ability to protect the confidentiality of our proprietary information; |
| • | risks associated with lawsuits to protect our patents or with respect to the infringement, misappropriations or other violations of intellectual property rights of third parties; |
| • | risks associated with failures to comply with our obligations under license agreements; |
| • | potential changes in patent law in the United States and other jurisdictions; |
| • | our ability to obtain and maintain our patent protection; |
19
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| • | impact of claims by third parties that we or our employees, consultants or independent contractors have infringed, misappropriated or otherwise violated their intellectual property; |
| • | our ability to protect our intellectual property and proprietary rights throughout the world; |
| • | our reliance on confidentiality agreements; |
| • | our ability to protect our trademarks and trade names; |
| • | threats not related to intellectual property; and |
| • | other risks addressed under the heading “Risk Factors” and elsewhere in this prospectus and in our Annual Report on Form 10-K for the year ended December 31, 2020, which is incorporated by reference herein. |
We derive many of our forward-looking statements from our operating budgets and forecasts, which are based on many detailed assumptions. While we believe that our assumptions are reasonable, we caution that it is very difficult to predict the impact of known factors, and it is impossible for us to anticipate all factors that could affect our actual results. Important factors that could cause actual results to differ materially from our expectations, or cautionary statements, are disclosed under the sections entitled “Risk Factors” in this prospectus and “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2020, which is incorporated by reference herein. All written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by these cautionary statements as well as other cautionary statements that are made from time to time in our other SEC filings and public communications. You should evaluate all forward-looking statements made in this prospectus or incorporated by reference herein in the context of these risks and uncertainties.
We caution you that the important factors referenced above may not contain all of the factors that are important to you. In addition, we cannot assure you that we will realize the results or developments we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our operations in the way we expect. The forward-looking statements included in this prospectus or incorporated by reference herein are made only as of the date hereof or thereof. We undertake no obligation to update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law.
20
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Unless otherwise indicated, information in this prospectus or incorporated by reference herein concerning economic conditions, our industry, our markets and our competitive position is based on a variety of sources, including information from independent industry analysts and publications, as well as our own estimates and research. This information involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While we are responsible for all disclosure in this prospectus or incorporated by reference herein, and we believe the information presented in this prospectus or incorporated by reference herein is generally reliable, forecasts, assumptions, expectations, beliefs, estimates and projects involve risk and uncertainties and are subject to change based on various factors, including those described under “Forward-Looking Statements” and “Risk Factors” in this prospectus and in our Annual Report on Form 10-K for the year ended December 31, 2020, which is incorporated by reference herein.
21
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
The selling stockholders are selling all of the Class A common stock being sold in this offering, including any shares sold upon the exercise of the underwriters’ option to purchase additional shares. See “Principal and Selling Stockholders”. Accordingly, we will not receive any proceeds from the sale of shares of our Class A common stock by the selling stockholders in this offering. We will bear the costs associated with the sale of the shares sold in this offering by the selling stockholders, other than underwriting discounts and commissions.
22
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
We currently intend to retain all available funds and any future earnings to fund the development and growth of our business and to repay indebtedness and, therefore, we do not anticipate paying any cash dividends in the foreseeable future. Additionally, because we are a holding company, our ability to pay dividends on our Class A common stock may be limited by restrictions on the ability of our subsidiaries to pay dividends or make distributions to us. Any future determination to pay dividends will be at the discretion of our Board, subject to compliance with covenants in current and future agreements governing our and our subsidiaries’ indebtedness, including our New Credit Agreement, and will depend on our results of operations, financial condition, capital requirements and other factors that our Board deems relevant.
Under the terms of the LLC Operating Agreement, Topco LLC is obligated to make tax distributions to current and future unitholders, including us and shall be made on a pro rata basis among the LLC Unitholders based on Topco LLC’s net taxable income and without regard to any applicable basis adjustment under Section 743(b) of the Code. These tax distributions may be substantial, and will likely exceed (as a percentage of Topco LLC’s income) the overall effective tax rate applicable to a similarly situated corporate taxpayer. As a result, it is possible that we will receive distributions significantly in excess of our tax liabilities and obligations to make payments under the Tax Receivable Agreement. While our Board may choose to distribute such cash balances as dividends on our Class A common stock (subject to the limitations set forth in the preceding paragraph), they will not be required to do so, and may in its sole discretion choose to use such excess cash for any purpose depending upon the facts and circumstances at the time of determination.
23
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Below is a list of the names, ages, positions, and a brief account of the business experience of the individuals who serve as executive officers and directors of Maravai as of March 24, 2021:
| Name |
Age |
Position | ||||
| Carl Hull |
63 | Chief Executive Officer and Director | ||||
| Eric Tardif |
52 | President | ||||
| Kevin Herde |
49 | Chief Financial Officer | ||||
| Brian Neel |
45 | Chief Operating Officer, Nucleic Acid Production | ||||
| Christine Dolan |
53 | Chief Operating Officer, Biologics Safety Testing | ||||
| Lisa V. Sellers |
49 | Chief Operating Officer, Protein Detection | ||||
| Kurt Oreshack |
41 | General Counsel and Secretary | ||||
| Anat Ashkenazi |
48 | Director | ||||
| Sean Cunningham |
45 | Director | ||||
| Benjamin Daverman |
43 | Director | ||||
| Susannah Gray |
60 | Director | ||||
| Robert B. Hance |
61 | Director | ||||
| Jessica Hopfield |
56 | Director | ||||
| Gregory T. Lucier |
56 | Director | ||||
| Luke Marker |
36 | Director | ||||
| Constantine Mihas |
54 | Director | ||||
| Murali K. Prahalad |
49 | Director | ||||
Carl Hull has served on our Board since November 2020 and has served as a member of MLSH 1’s board since March 2016. Mr. Hull brings over 35 years of sales, marketing and general management leadership in the diagnostics and life sciences industries. From 2009 to 2012, Mr. Hull was Chief Executive Officer of Gen-Probe Incorporated (“Gen-Probe”), a medical diagnostics company, and served as its Chief Operating Officer from 2007 to 2009. Under Mr. Hull’s leadership, Gen-Probe took full advantage of its core molecular diagnostics and automation strengths and launched several highly innovative products including the PANTHER® molecular diagnostic system and APTIMA® HPV screening assay. During Mr. Hull’s tenure, Gen-Probe extended its recognized leadership position in the most rapidly growing diagnostics market segment and the market capitalization of Gen-Probe doubled, creating nearly $2 billion in value for shareholders and culminating in a successful sale to Hologic in 2012. Prior to Gen-Probe, Mr. Hull had been in sales, marketing and management positions for Abbott Laboratories, Ventana Medical Systems, Inc. (acquired by Roche Holding AG), Applied Imaging Corp. (now part of Danaher Corporation) and Applied Biosystems Inc. (now part of Thermo Fisher Scientific Inc. (“Thermo Fisher”)), all biomedical technology companies. Mr. Hull serves as Chairman of the Board for The Binding Site and is a member of the Board of Ortho Clinical Diagnostics, both leading human diagnostics companies. Mr. Hull holds an MBA from the University of Chicago and a BA in Political Science and International Relations from the Johns Hopkins University. Mr. Hull is a valuable member of our Board due to his experience as our Chief Executive Officer, his executive experience at other biomedical technology companies and his experience as an executive at a publicly-traded company.
Eric Tardif has served as our President since he co-founded Maravai in March 2014. Prior to co-founding Maravai, he led corporate development and corporate strategy at Gen-Probe Incorporated. Following the acquisition of Gen-Probe by Hologic, Inc. (“Hologic”), a medical technology company, in 2012,
24
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Mr. Tardif was promoted to lead corporate strategy for Hologic. Mr. Tardif began his career as an investment banker executing mergers and acquisitions at investment banking firms Merrill Lynch, Piper Jaffray and Morgan Stanley, with a focus on medical device companies, particularly in the life sciences tools and diagnostics segments. Mr. Tardif has a Master of Science in Finance from Boston College, an MBA from University of British Columbia and a BA in Business Administration from Major Bishops University.
Kevin Herde has served as our Chief Financial Officer since May 2017. Prior to joining Maravai, he served as Executive Vice President and Chief Financial Officer at Sorrento Therapeutics, Inc., a biopharmaceutical company, from April 2016 to May 2017 and as Vice President of Global Blood Screening at Hologic from January 2015 to February 2016. Mr. Herde also served as Vice President, Finance and Corporate Controller at Gen-Probe prior to its acquisition by Hologic in 2012. Mr. Herde began his career at KPMG LLC. Mr. Herde holds a BBA in Business Administration from University of San Diego and is a certified public accountant in California (inactive).
Brian Neel has served as the Chief Operating Officer for our Nucleic Acid Production business segment since October 2017. Prior to joining Maravai, Mr. Neel was Vice President of Operations of Codex DNA, Inc. (formerly Synthetic Genomics DNA) (“Codex”), a biological equipment company, from May 2016 to October 2017. Prior to joining Codex, Mr. Neel was Vice President of Operations of GenMark Diagnostics, Inc. (“Genmark”), a molecular diagnostics company, from 2014 to 2016. Prior to joining GenMark, Mr. Neel was the Site Manufacturing Operations Leader at Thermo Fisher Scientific (formerly Life Technologies) from January 2013 to June 2014. Prior to joining Thermo Fisher, Mr. Neel was a Global Operations Associate Director and Manufacturing Operations Leader at Life Technologies, Inc. (“Life Technologies”), a global life sciences company that was ultimately purchased by Thermo Fisher in 2014, for over eleven years. Mr. Neel holds a BS in Microbiology from the University of Missouri.
Christine Dolan has served as the Chief Operating Officer of our Biologics Safety Testing business segment since October 2017. Prior to joining Maravai, Ms. Dolan held several operational and business leadership roles including Senior Vice President of Product Development, VP of Global Operations and VPGM of Development and Analytical Services at Catalent Pharma Solutions, where Ms. Dolan worked for over eight years. Prior to joining Catalent Pharma Solutions, Ms. Dolan was Director of Nuclear Operations and Global Quality Control at GE Healthcare and Amersham Health where she worked in progressive management roles for a total of over thirteen years. Ms. Dolan holds a BS in Biology from Lenoir-Rhyne College.
Lisa V. Sellers has served as the Chief Operating Officer of our Protein Detection business segment since August 2020. With over 20 years of experience, Dr. Sellers is an experienced general manager and commercial executive. Prior to joining Maravai, Dr. Sellers was Vice President of Marketing at 10X Genomics, Inc. (“10X”). Prior to 10X, Dr. Sellers led global reagent and instrumentation businesses within Applied Biosystems, Life Technologies and then Thermo Fisher Scientific. While at Thermo Fisher, Dr. Sellers also led B2B business development and sales channels to supply and out-license a portfolio of genetic analysis products and IP to the molecular diagnostic market. Dr. Sellers received her PhD in Chemistry from the University of Colorado at Boulder and her BS in Chemistry from Santa Clara University.
Kurt Oreshack has served as our General Counsel since November 2020. Prior to joining Maravai, Mr. Oreshack was a partner in the law firm of Breakwater Law Group, LLP in Solana Beach, CA, practicing in the field of corporate and securities law. Prior to joining Breakwater Law Group, Mr. Oreshack was the General Counsel of Human Longevity, Inc., a San Diego-based genomic research and in vitro diagnostics company, from June 2015 through September 2017. After leaving Human Longevity, Mr. Oreshack practiced law individually and as General Counsel in Residence at the law firm of Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP in San Diego, CA until joining Breakwater Law Group in January 2019. Mr. Oreshack received a JD from the University of Notre Dame Law School and a BA from Loyola University Chicago. He is a member of the State Bar of California.
25
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Anat Ashkenazi has served on our Board and its Audit Committee since our IPO in November 2020. Ms. Ashkenazi is the Chief Financial Officer of Eli Lilly and Company, where she has worked for over nineteen years. Ms. Ashkenazi joined Eli Lilly in 2001 and has had a diverse career spanning financial, strategy and operations roles. Prior to her current position, Ms. Ashkenazi held roles as the Senior Vice President of Finance of Eli Lilly and as chief financial officer for a number of global divisions within Eli Lilly, including Oncology, Diabetes, Global Manufacturing & Quality and Research & Development. Ms. Ashkenazi holds an MBA from Tel Aviv University and a BA in Economics & Business Administration from the Hebrew University. We determined that Ms. Ashkenazi’s extensive executive experience in the pharmaceutical industry, as well as her financial expertise, qualifies her to serve as a director on the Board.
Sean Cunningham has served on our Board since our IPO in November 2020 and has served as a member of MLSH 1’s board since March 2016. Mr. Cunningham joined GTCR in 2001 where he is currently a Managing Director. He was previously a consultant with The Boston Consulting Group. Mr. Cunningham holds an MBA from the Wharton School at the University of Pennsylvania as well as a BA and BE in Engineering sciences from Dartmouth College. We determined that Mr. Cunningham’s directorship experience with similar companies and extensive experience in the healthcare and pharmaceutical industries qualifies him to serve as a director on the Board.
Benjamin Daverman has served on our Board and its Compensation and Nominating Committee since our IPO in November 2020 and has served as a member MLSH 1’s board since March 2016. Mr. Daverman joined GTCR in 2008 where he is currently a Managing Director. Prior to joining GTCR, he worked as a Venture Capitalist at Alta Partners, a venture capital firm, as well as an Investment Banking Associate at JMP Securities and an Analyst in the mergers and acquisitions group at J.P. Morgan (formerly Hambrecht & Quist), both investment banking firms. Mr. Daverman holds an MBA from the Wharton School at the University of Pennsylvania and a BA in History from Colgate University. He also holds an MS in Biotechnology from the School of Engineering and Applied Science at the University of Pennsylvania. We determined that Mr. Daverman’s extensive directorship experience with similar companies, and extensive experience in the healthcare, pharmaceutical and life sciences qualifies him to serve as a director on the Board.
Susannah Gray has served on our Board and as the Chairwoman of its Audit Committee since our IPO in November 2020. Ms. Gray served as the Chief Financial Officer of Royalty Pharma Management LLC (“Royalty Pharma”), a buyer of pharmaceutical royalties, from January 2005 to December 2018. She was promoted to Executive Vice President of Finance and Strategy in December 2018 and retired from Royalty Pharma in September 2019. Prior to Royalty Pharma, Ms. Gray served as a managing director and senior analyst covering the healthcare sector of CIBC World Markets’ high yield group from 2002 to 2004, and also previously served in similar roles at Merrill Lynch and Chase Securities (predecessor of J.P. Morgan Securities). Ms. Gray holds an MBA from Columbia University and a BA in Social Studies from Wesleyan University. We determined that Ms. Gray’s extensive executive experience in the pharmaceutical industry, as well as her financial expertise, qualifies her to serve as a director on the Board.
Robert B. Hance has served on our Board since our IPO in November 2020 and has served as a member of MLSH 1’s board since 2017. Mr. Hance is a medical device industry veteran with more than 25 years’ experience and has served as the Chief Executive Officer of Regatta Medical, Inc. (“Regatta Medical”), a medical device company, since 2017. Prior to Regatta Medical from 2013 to 2016, Mr. Hance was Chief Executive Officer of Creganna Medical Devices, Inc. (“Creganna Medical”), a leading supplier to the minimally invasive medical device industry. Creganna Medical was sold to TE Connectivity Ltd. in 2016. From 2012 to 2013, Mr. Hance was an Entrepreneur-in-Residence within the FDA at the Center for Devices and Radiological Health. Prior to his FDA experience, Mr. Hance was President of Abbott Vascular, the cardiovascular device division of Abbott Laboratories, a biomedical company. Mr. Hance holds an MBA from Harvard Business School and a BS in Chemical Engineering from the Massachusetts Institute of Technology. We determined Mr. Hance’s extensive expertise in the medical device and life sciences industry qualifies him to serve as a director on the Board.
26
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Jessica Hopfield, PhD has served on our Board and its Audit Committee and Compensation and Nominating Committee since our IPO in November 2020. Dr. Hopfield is a scientist and business leader with more than two decades of experience in the medical and healthcare fields. She serves as an independent director on the Board of Directors of Insulet Corporation and Editas Medicine. In addition, she is a strategic advisor and investor in start-up healthcare firms. Dr. Hopfield is a former Partner of McKinsey & Company in its global pharmaceuticals and medical devices practice where she led work in strategy, research and development management, and marketing across pharmaceutical, biotechnology and medical device industries. She also held management positions at Merck Sharp & Dohme Corp., a pharmaceutical company, in clinical development, outcomes research, and marketing. Dr. Hopfield holds a PhD in Biological Sciences from The Rockefeller University, an MBA from Harvard Business School and a BS in Biology from Yale College. We determined that Dr. Hopfield is qualified to serve as a member of our Board because of her extensive experience in the life sciences industry, educational background and service as an independent director to other public companies.
Gregory T. Lucier has served on our Board since our IPO in November 2020 and has served as a member of MLSH 1’s board since January 2020. Mr. Lucier has served as the Chief Executive Officer of Corza Health, Inc. (“Corza Health”), a life sciences company, since 2018 and is a 25-year veteran of the healthcare industry. Prior to Corza Health, Mr. Lucier was Chairman and Chief Executive Officer of NuVasive, Inc. (“NuVasive”) from 2015 to 2018. NuVasive is an innovative medical device company specializing in minimally invasive spine surgery. Prior to NuVasive, from 2003 to 2014, Mr. Lucier served as Chairman and CEO of Life Technologies. Mr. Lucier’s early career included roles as a corporate officer of General Electric Company and as an executive at GE Medical Systems Information Technologies, Inc., a healthcare company. Mr. Lucier serves as a director of Catalent, a global pharmaceutical products manufacturer, Dentsply Sirona Inc., a global provider of professional dental products and technologies, and Berkeley Lights, a life sciences company. He has an MBA from Harvard Business School and a BA in Industrial Engineering from Pennsylvania State University. We determined Mr. Lucier’s extensive experience in the healthcare and medical device industry, in addition to his experience on multiple public and private boards of directors, qualifies him to serve as a director on the Board.
Luke Marker has served on our Board since our IPO in November 2020 and has served as a member of MLSH 1’s board since 2016. Mr. Marker joined GTCR in 2009 where he is currently a Principal. Prior to joining GTCR, he worked in the investment banking division at Lehman Brothers and Barclays Capital. Mr. Marker holds an MBA with distinction from Harvard Business School and a BA in Mathematics and Economics from Kalamazoo College. We determined that Mr. Marker’s directorship experience with similar companies and extensive experience in the healthcare, pharmaceutical and life sciences industries qualifies him to serve as a director on the Board.
Constantine Mihas has served on our Board and as Chairman of its Compensation and Nominating Committee since our IPO in November 2020 and has served as a member MLSH 1’s board since March 2016. Mr. Mihas joined GTCR in 2001 where he is currently a Managing Director and head of the Healthcare group. Prior to joining GTCR, Mr. Mihas was Chief Executive Officer and co-founder of Delray Farms, LLC (“Delray Farms”), a specialty food retailer. Prior to Delray Farms, Mr. Mihas was with McKinsey & Company, Inc., a consulting firm. Mr. Mihas holds an MBA with distinction from the Harvard Business School and a BS in Finance and Economics from the University of Illinois, Chicago. We determined that Mr. Mihas’s directorship experience with similar companies, deep business background, and extensive experience in the healthcare, pharmaceutical and life sciences industries qualifies him to serve as a director on the Board.
Murali K. Prahalad has served on our Board since our IPO in November 2020 and has served as a member MLSH 1’s board since August 2016. Dr. Prahalad is currently the President and Chief Executive Officer of Iridia, Inc., a nanotechnology company, and was most recently the President and Chief Executive Officer of Epic Sciences, Inc., a medical diagnostics company, from August 2013 through April 2019. Dr. Prahalad has two decades of experience in the technology and life science industries. From 2007 through 2013, Dr. Prahalad served in multiple roles at Life Technologies, including as Vice President of Corporate Strategy. Before Life
27
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Technologies, Dr. Prahalad was Vice President of Business Development at Sequenom, Inc., a biotechnology company. Dr. Prahalad received a PhD in biochemistry and molecular pharmacology as well as an MS in medical sciences from Harvard University. He also holds a BS in Cellular and Molecular Biology and Economics from the University of Michigan. We determined Dr. Prahalad’s extensive experience in the technology and life sciences industry, in addition to his medical expertise and experience on boards of directors, qualifies him to serve as a director on the Board.
Family Relationships
There are no family relationships between any of our executive officers or directors.
Corporate Governance
Board Composition and Director Independence
Our business and affairs are managed under the direction of our Board. Our Board is composed of 11 directors. Our certificate of incorporation provides that the authorized number of directors may be changed only by resolution of our Board. In addition, the Director Nomination Agreement prohibits us from increasing or decreasing the size of our Board without the prior written consent of GTCR. Our certificate of incorporation also provides that our Board is divided into three classes of directors, with the classes as nearly equal in number as possible. Subject to any earlier resignation or removal in accordance with the terms of our certificate of incorporation and bylaws, our Class I directors are Messrs. Hull, Daverman and Mihas and Ms. Gray and are serving until the first annual meeting of shareholders following the IPO, our Class II directors are Messrs. Cunningham and Hance and Drs. Hopfield and Prahalad and are serving until the second annual meeting of shareholders following the IPO and our Class III directors are Messrs. Lucier and Marker and Ms. Ashkenazi and are serving until the third annual meeting of shareholders following the IPO. This classification of our Board could have the effect of increasing the length of time necessary to change the composition of a majority of the Board. In general, at least two annual meetings of shareholders will be necessary for shareholders to effect a change in a majority of members of the Board. In addition, our certificate of incorporation provides that our directors may be removed with or without cause by the affirmative vote of at least a majority of the voting power of our outstanding shares of stock entitled to vote thereon, voting together as a single class for so long as GTCR beneficially owns 40% or more, in the aggregate, of the total number of shares of our common stock then outstanding. If GTCR’s aggregate beneficial ownership falls below 40% of the total number of shares of our common stock outstanding, then our directors may be removed only for cause upon the affirmative vote of at least 66 2/3% of the voting power of our outstanding shares of stock entitled to vote thereon.
In addition, at any time when GTCR has the right to designate at least one nominee for election to our Board, GTCR also has the right to have one of its nominated directors hold one seat on each Board committee, subject to satisfying any applicable stock exchange rules or regulations regarding the independence of Board committee members. The listing standards of NASDAQ require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act.
Our Board has also determined that Messrs. Hance and Lucier, Ms. Ashkenazi, Ms. Gray and Drs. Hopfield and Prahalad meet the requirements to be independent directors. In making this determination, our Board considered the relationships that each such non-employee director has with Maravai and all other facts and circumstances that our Board deemed relevant in determining their independence, including beneficial ownership of our common stock.
Controlled Company Status
After completion of this offering, GTCR will continue to control a majority of the voting power in us. As a result, we will continue to be a “controlled company.” Under NASDAQ rules, a company of which more than
28
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including the requirements that, within one year of the date of the listing of our common stock:
| • | we have a board of directors that is composed of a majority of “independent directors,” as defined under the rules of such exchange; |
| • | we have a compensation committee that is composed entirely of independent directors; and |
| • | we have a nominating and corporate governance committee that is composed entirely of independent directors. |
We will continue to rely on this exemption. As a result, we may not have a majority of independent directors on our Board. In addition, our Compensation Committee and our Nominating and Corporate Governance Committee may not consist entirely of independent directors or be subject to annual performance evaluations. Accordingly, you may not have the same protections afforded to shareholders of companies that are subject to all of the NASDAQ corporate governance requirements.
Board Committees
Our Board has an Audit Committee and a Compensation and Nominating Committee. The composition, duties and responsibilities of these committees are as set forth below. In the future, our Board may establish other committees, as it deems appropriate, to assist it with its responsibilities.
| Board Member |
Audit Committee | Compensation and Nominating Committee |
||||||
| Carl Hull |
||||||||
| Anat Ashkenazi |
X | |||||||
| Sean Cunningham |
||||||||
| Benjamin Daverman |
X | |||||||
| Susannah Gray |
X (Chair) | |||||||
| Robert B. Hance |
||||||||
| Jessica Hopfield |
X | X | ||||||
| Gregory T. Lucier |
||||||||
| Luke Marker |
||||||||
| Constantine Mihas |
X (Chair) | |||||||
| Murali K. Prahalad |
||||||||
Audit Committee
Our Audit Committee is composed of Dr. Hopfield, Ms. Gray and Ms. Ashkenazi, with Ms. Gray serving as chairman of the committee. We comply with the audit committee requirements of the SEC and NASDAQ, which require that the Audit Committee be composed of at least one independent director at the closing of the IPO, a majority of independent directors within 90 days following this offering and all independent directors within one year following the IPO. Our Board has determined that Dr. Hopfield, Ms. Gray and Ms. Ashkenazi meet the independence requirements of Rule 10A-3 under the Exchange Act and the applicable listing standards of NASDAQ. Our Board has determined that Ms. Gray and Ms. Ashkenazi are “audit committee financial experts” within the meaning of SEC regulations and applicable listing standards of NASDAQ. The Audit Committee’s responsibilities include:
| • | appointing, approving the compensation of, and assessing the qualifications, performance and independence of our independent registered public accounting firm; |
| • | pre-approving audit and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
29
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| • | discusses on a periodic basis, or as appropriate, with management, our policies, programs and controls with respect to risk assessment and risk management; |
| • | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us; |
| • | is responsible for reviewing our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; |
| • | monitors the rotation of partners of the independent registered public accounting firm on our engagement team in accordance with requirements established by the SEC; |
| • | reviews management’s report on its assessment of the effectiveness of internal control over financial reporting and any changes thereto; |
| • | reviewing the adequacy of our internal control over financial reporting; |
| • | establishing policies and procedures for the receipt, retention, follow-up and resolution of accounting, internal controls or auditing matters, complaints and concerns; |
| • | recommending, based upon the Audit Committee’s review and discussions with management and the independent registered public accounting firm, whether our audited financial statements shall be included in our Annual Report on Form 10-K; |
| • | monitoring our compliance with legal and regulatory requirements as they relate to our consolidated financial statements and accounting matters; |
| • | preparing the Audit Committee report required by the rules of the SEC to be included in our annual proxy statement; |
| • | annually reviews and assesses treasury functions including cash management process; |
| • | investigates any matters received, and reports to the Board periodically, with respect to ethics issues, complaints and associated investigations; |
| • | reviews the audit committee charter and the committee’s performance at least annually; |
| • | consults with management to establish procedures and internal controls relating to cybersecurity; |
| • | reviewing all related party transactions for potential conflict of interest situations and approving all such transactions; and |
| • | reviewing and discussing with management our earnings releases and scripts. |
Compensation and Nominating Committee
Our Compensation and Nominating Committee is composed of Messrs. Mihas and Daverman and Dr. Hopfield, with Mr. Mihas serving as chairman of the committee. The Compensation and Nominating Committee’s responsibilities include:
| • | annually reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer; |
| • | evaluating the performance of our chief executive officer in light of such corporate goals and objectives and determining and approving the compensation of our chief executive officer; |
| • | reviewing and approving the compensation of our other executive officers; |
| • | appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
30
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| • | conducting the independence assessment outlined in NASDAQ rules with respect to any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
| • | annually reviewing and reassessing the adequacy of the committee charter in its compliance with the listing requirements of NASDAQ; |
| • | reviewing and establishing our overall management compensation, philosophy and policy; |
| • | overseeing and administering our compensation and similar plans; |
| • | reviewing and making recommendations to our Board with respect to director compensation; |
| • | reviewing and discussing with management the compensation discussion and analysis to be included in our annual proxy statement or Annual Report on Form 10-K; |
| • | developing and recommending to our Board criteria for board and committee membership; |
| • | subject to the rights of GTCR under the Director Nomination Agreement as described in “Certain Relationships and Related Party Transactions—Director Nomination Agreement,” identifying and recommending to our Board the persons to be nominated for election as directors and to each of our Board’s committees; |
| • | developing and recommending to our Board best practices and corporate governance principles; |
| • | developing and recommending to our Board a set of corporate governance guidelines; and |
| • | reviewing and recommending to our Board the functions, duties and compositions of the committees of our Board. |
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past fiscal year has served, as a member of the Board or compensation committee of any entity that has one or more executive officers serving on our Board or Compensation and Nominating Committee.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Our code of business conduct and ethics is available on our website. We intend to disclose any amendments to the code, or any waivers of its requirements, on our website.
31
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
EXECUTIVE AND DIRECTOR COMPENSATION
The following section provides compensation information pursuant to the scaled disclosure rules applicable to “emerging growth companies” under the rules of the SEC and may contain statements regarding future individual and company performance targets and goals. These targets and goals should not be understood to be statements of management’s expectations or estimates of results or other guidance. We specifically caution investors not to apply these statements to other contexts.
We are currently considered an “emerging growth company” within the meaning of the Securities Act for purposes of the SEC’s executive compensation disclosure rules. Accordingly, we are required to provide a Summary Compensation Table and an Outstanding Equity Awards at Fiscal Year-End Table, as well as limited narrative disclosures regarding executive compensation for our last completed fiscal year. Further, our reporting obligations extend only to the following “Named Executive Officers,” who are the individuals who served as our principal executive officer during 2020 and the next two most highly compensated executive officers at the end of the fiscal year ended December 31, 2020. For the fiscal year ended December 31, 2020, our Named Executive Officers and their principal positions were as follows:
| Named Executive Officer |
Principal Position | |
| Carl Hull |
Chief Executive Officer | |
| Eric Tardif |
President and Executive Vice-President, Corporate Development | |
| Brian Neel |
Chief Operating Officer, Nucleic Acid Production | |
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt in the future may differ materially from the currently planned programs summarized in this discussion.
Summary Compensation Table
| Name and principal position |
Year | Salary | Option Awards(1) |
Nonequity incentive plan compensation(2) |
All Other Compensation(3) |
Total | ||||||||||||||||||
| Carl Hull, Chief Executive Officer |
2020 | $ | 500,000 | $ | 874,160 | $ | 750,000 | — | $ | 2,124,160 | ||||||||||||||
| 2019 | $ | 485,417 | $ | 1,743,000 | $ | 600,000 | — | $ | 2,828,417 | |||||||||||||||
| Eric Tardif, President and Executive Vice-President, Corporate Development(4) |
2020 | $ | 404,882 | $ | 874,160 | $ | 608,756 | $ | 8,550 | $ | 1,895,934 | |||||||||||||
| Brian Neel, Chief Operating Officer, Nucleic Acid Production |
2020 | $ | 333,238 | $ | 874,160 | $ | 649,943 | $ | 8,550 | $ | 1,860,601 | |||||||||||||
| 2019 | $ | 314,673 | $ | 428,000 | $ | 142,436 | $ | 8,400 | $ | 893,509 | ||||||||||||||
| (1) | The amounts reported in the Option Awards column represent the grant date fair value of, for 2020, stock options with respect to our Class A common stock and, for 2019, the incentive units in MLSH 1, in each case, granted to the Named Executive Officers as computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, Compensation – Stock Compensation. The assumptions used in calculating the grant date fair value of the stock options with respect to our Class A common stock and the incentive units in MLSH 1 reported in the Option Awards column are set forth in Note 10 to the consolidated financial statements included in our Annual Report Form 10-K for the year ended December 31, 2020, incorporated by reference in this prospectus. The amounts reported in this |
32
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| column reflect the accounting cost for these incentive units and stock options and do not correspond to the actual economic value that may be received by the Named Executive Officers for the incentive units. See “Narrative Disclosure to Summary Compensation Table—Equity Incentives” below for additional details. |
| (2) | The amounts reported in the Non-Equity Incentive Plan Compensation column reflect bonuses paid to the Named Executive Officers under the Bonus Plan (as defined below), for 2020, with respect to the fiscal year ended December 31, 2020 and for 2019, with respect to the fiscal year ended December 31, 2019 . Please see the section entitled “Narrative Disclosure to Summary Compensation Table—Employment Agreements” below for additional details. |
| (3) | The amounts reported in the All Other Compensation column reflect 401(k) plan matching contributions made on behalf of the Named Executive Officers, for 2020, during the fiscal year ended December 31, 2020 and, for 2019, during the fiscal year ended December 31, 2019. See below under “Additional Narrative Disclosure—Retirement Benefits” for additional information regarding 401(k) plan contributions. |
| (4) | Mr. Tardif was not a named executive officer for the fiscal year ended December 31, 2019; therefore, we did not include his 2019 compensation. |
Narrative Disclosure to Summary Compensation Table
Employment Agreements
We have entered into employment agreements with each of our Named Executive Officers that provide for annual base salary, target bonus opportunity, paid vacation, reimbursement of reasonable business expenses and eligibility to participate in our benefit plans generally.
Messrs. Hull’s, Tardif’s and Neel’s annualized base salaries at the end of the 2020 fiscal year were $500,000, $404,468 and $327,948, respectively, and their target annual bonuses were 100%, 75% and 40% of base salary. For the 2020 fiscal year, Messrs. Hull, Tardif and Neel received bonus payments (inclusive of the amounts payable pursuant to the supplemental bonus plan described below) of $750,000, $608,756 and $649,943, respectively, based in part on pre-established company performance metrics and based in part on individual achievement. The pre-established company performance metrics for the 2020 fiscal year consisted of adjusted revenue (weighted 30%), Adjusted EBITDA (weighted 50%), and achievement of corporate initiatives (weighted 20%). Additionally, the Board approved a supplemental bonus plan for fiscal year 2020, which was funded based on the Company’s achievement of gross EBITDA above certain thresholds. Mr. Tardif and Mr. Neel received supplemental bonus payments of $150,000 and $450,000, respectively. For the 2020 fiscal year, we achieved the pre-established company performance metrics at 172% of target under our original bonus plan and 245% of target under our supplemental bonus plan. Under our original bonus plan, the company performance metric score was then adjusted based on individual achievement to yield total achievement scores, which corresponded to payouts of 150% of target for Mr. Hull, 151% of target for Mr. Tardif and 152% of target for Mr. Neel.
The employment agreements also provide for certain severance benefits upon a resignation by the applicable Named Executive Officer for “good reason” or upon a termination by the Company without “cause.” Please see the section entitled “Additional Narrative Disclosure—Potential Payments Upon Termination or Change in Control” below for more details regarding the severance benefits provided to our Named Executive Officers under the employment agreements.
Equity Incentives
Incentive Units
Historically, we have offered equity incentives to our Named Executive Officers through grants of incentive units in MLSH 1. Certain of these incentive unit awards are subject to time-based vesting requirements and are subject to accelerated vesting upon the occurrence of certain terminations of employment and certain change-in-control events, and the remaining incentive unit awards are subject to market and performance-based
33
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
vesting requirements and terminate if such performance-based vesting requirements are not met upon certain change-in-control events. As anticipated, the consummation of our IPO did not trigger accelerated vesting of any of the incentive units in MLSH 1 that are subject to time-based vesting requirements; however, as we expected the vesting of the incentive units subject to performance-based vesting requirements were accelerated as a result of the IPO. See below under “—Additional Narrative Disclosure—Potential Payments Upon Termination or Change in Control” for additional information regarding the circumstances that could result in accelerated vesting of these awards.
Stock Options
In connection with our IPO, we granted certain employees, including our Named Executive Officers, stock options with respect to our Class A common stock pursuant to our 2020 Omnibus Incentive Plan. These stock option awards vest over four years with 25% vesting on the first anniversary of the grant date and the remaining portion of the award vesting monthly over the three-year period thereafter, subject to the recipient’s continued employment through each vesting date. These stock options were issued with an exercise price equal to the IPO price of $27.00 per share. See below under “—Additional Narrative Disclosure—Potential Payments Upon Termination or Change in Control” for additional information regarding the circumstances that could result in accelerated vesting of these awards.
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes, for each of the Named Executive Officers, the number of stock options with respect to our Class A common stock and incentive units in MLSH 1 held as of December 31, 2020.
| Name |
Grant date |
Number of securities underlying unexercised options (#) exercisable |
Number of securities underlying unexercised options (#) unexercisable |
Option exercise price ($) |
Option expiration date |
|||||||||||||||
| Carl Hull |
||||||||||||||||||||
| Stock Options |
11/19/2020 | 60,000 | (2) | 27.00 | 11/19/2030 | |||||||||||||||
| MLSH 1 Incentive Units |
3/18/2014 | 312,540.72 | 80,000 | (3) | N/A | (7) | N/A | (7) | ||||||||||||
| MLSH 1 Incentive Units |
6/20/2019 | 44,000 | 56,000 | (4) | N/A | (7) | N/A | (7) | ||||||||||||
| Eric Tardif |
||||||||||||||||||||
| Stock Options |
11/19/2020 | 60,000 | (2) | 27.00 | 11/19/2030 | |||||||||||||||
| MLSH 1 Incentive Units |
3/18/2014 | 177,016.29 | 40,000 | (3) | N/A | (7) | N/A | (7) | ||||||||||||
| Brian Neel |
||||||||||||||||||||
| Stock Options |
11/19/2020 | 60,000 | (2) | 27.00 | 11/19/2030 | |||||||||||||||
| MLSH 1 Incentive Units |
12/27/2017 | 51,000 | 14,000 | (5) | N/A | (7) | N/A | (7) | ||||||||||||
| MLSH 1 Incentive Units |
12/13/2019 | 4,000 | 16,000 | (6) | N/A | (7) | N/A | (7) | ||||||||||||
| (1) | This table reflects information regarding stock options with respect to our Class A common stock and incentive units in MLSH 1 granted to our Named Executive Officers that were outstanding as of December 31, 2020. For more information on these stock options and incentive units, see “Narrative Disclosure to Summary Compensation Table—Equity Incentives” above. |
| (2) | Under the terms of the applicable stock option award agreement, these stock options vest 25% on the first anniversary of the grant date and monthly thereafter over the next three years subject to such executive’s continued employment through each vesting date. |
| (3) | Under the terms of the applicable incentive unit award documentation, these incentive units will vest on April 5, 2021, so long as Mr. Hull or Mr. Tardif, as applicable, remains employed through such date, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
34
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| (4) | Under the terms of the applicable incentive unit award documentation, these incentive units will vest in equal installments on June 20 of each of 2021, 2022, 2023 and 2024, so long as Mr. Hull remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (5) | Under the terms of the applicable incentive unit award documentation, these incentive units will vest in equal installments on October 16 of each of 2021 and 2022, so long as Mr. Neel remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (6) | Under the terms of the applicable incentive unit award documentation, these incentive units will vest in equal installments on December 13 of each of 2021, 2022, 2023 and 2024, so long as Mr. Neel remains employed through such dates, and vesting of such incentive units accelerates upon a qualifying sale of MLSH 1. |
| (7) | These equity awards are not traditional options and, therefore, there is no exercise price or option expiration date associated with them. |
Additional Narrative Disclosure
Retirement Benefits
We do not have a defined benefit pension plan or nonqualified deferred compensation plan. We currently maintain a retirement plan intended to provide benefits under Section 401(k) of the Code, pursuant to which employees, including the Named Executive Officers, can make voluntary pre-tax contributions. We match 50% of elective deferrals up to 6% of elective deferrals for all participants. These matching contributions are vested or vest based on the participant’s length of service with us, becoming fully vested on the fourth anniversary of the participant’s date of hire. All contributions under the plan are subject to certain annual dollar limitations, which are periodically adjusted for changes in the cost of living.
Employee Stock Purchase Plan
We also maintain the 2020 Employee Stock Purchase Plan (“ESPP”). The ESPP authorizes the grant of options to employees, which due to our “Up-C” structure is not currently tax-qualified under Section 423 of the Code. Each offering period is approximately twenty-four months in duration commencing on each May 1 and November 1 of each year during the term of the ESPP. The ESPP allows participants to purchase common stock through payroll deductions of up to 15% of their eligible compensation. The purchase price of the shares will be 85% of the lower of the fair market value of our common stock on the grant date or purchase date. As of December 31, 2020, no shares of common stock have been purchased under our ESPP.
Potential Payments Upon Termination or Change in Control
A Named Executive Officer’s outstanding incentive units in MLSH 1 that vest based on time will become 100% vested upon a “sale” of MLSH 1, which is generally the sale of (i) MLSH 1’s equity securities pursuant to which an independent third party or parties acquires a majority of the equity securities or voting power to elect a majority of the board of directors of MLSH 1 or (ii) all or substantially all of MLSH 1’s assets on a consolidated basis.
Stock options held by our Named Executive Officers will become 100% vested and exercisable if such executive’s employment is terminated by us without “cause” or by the executive for “good reason”, each as defined in the executive’s employment agreement, within one year following a “change in control” of the Company, as defined in our 2020 Omnibus Incentive Plan
Our Named Executive Officers’ employment agreements provide that upon a termination by us for any reason other than for “cause” or upon a resignation by such Named Executive Officer for “good reason,” each as defined therein, subject to the execution and delivery of a fully effective release of claims, as prepared by the Company, and continued compliance with applicable restrictive covenants, Mr. Hull and Mr. Tardif will receive
35
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
salary continuation payments equal to 100% of base salary, a prorated target bonus, and COBRA premium reimbursement for 12 months, and Mr. Neel will receive salary continuation payments equal to 75% of base salary and COBRA premium reimbursement for 9 months. If such conditions occur during the 12-month period following a “change in control” of the Company, as defined therein, then Mr. Hull will receive salary continuation payments equal to 200% of base salary, plus an amount equal to two times his target annual bonus, and COBRA premium reimbursement for up to 18 months, and Mr. Neel and Mr. Tardif will receive salary continuation payments equal to 100% of base salary plus, for Mr. Tardif, his target annual bonus, and COBRA premium reimbursement for up to 12 months.
The employment agreements also contain certain restrictive covenants, including provisions that create restrictions, with certain limitations, on our Named Executive Officers competing with Maravai and its subsidiaries during the term of the Named Executive Officer’s employment with the Company and soliciting any customers or other business relations or soliciting or hiring employees of Maravai and its subsidiaries, in each case, during the term of the Named Executive Officer’s employment with the Company and for the one-year period following termination of employment.
Non-Employee Director Compensation Policy
Prior to the IPO, we did not have a formal policy with respect to compensating our non-employee directors for service as directors. Prior to the IPO, each of Messrs. Hance, Lucier and Prahalad was subject to an investment and director compensation agreement with MLSH 1, pursuant to which they have been granted restricted incentive units in MLSH 1 and were entitled to director fees ($10,000 per meeting attended in person and $5,000 per meeting attended telephonically) and reimbursement of expenses incurred in connection with their service.
Since the completion of the IPO, our non-employee directors have been eligible to receive the annual cash retainers listed below for their service on our Board. The non-employee directors who are employees of GTCR or its affiliates have agreed or are otherwise obligated to transfer all or a portion of the compensation they receive for their service as directors to GTCR or its affiliates. The retainers will be paid in four equal quarterly installments and prorated for any partial year of service on our board of directors.
| Position |
Retainer ($) | |||
| Board Member |
40,000 | |||
| Audit Committee: |
||||
| Chairperson |
20,000 | |||
| Committee Member |
10,000 | |||
| Compensation and Nominating Committee: |
||||
| Chairperson |
25,000 | |||
| Committee Member |
12,500 | |||
All non-employee directors are also reimbursed for their reasonable expenses to attend meetings of our Board and related committees and otherwise attend to our business.
36
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
The following table presents the total compensation for each person who served as a non-employee member of our Board during 2020. Other than as set forth in the table and described more fully below, we did not pay any compensation, reimburse any expense of, make any equity awards or non-equity awards to, or pay any other compensation to, any of the other non-employee members of our Board in 2020. Mr. Hull, our Chief Executive Officer, receives no compensation for service as a director and, consequently, is not included in this table. The compensation received by Mr. Hull as an employee of the Company is presented in “—Summary Compensation Table.”
Director Compensation
| Name |
Fees earned or paid in cash ($)(1) |
Stock awards ($)(2) |
Total ($) |
|||||||||
| Anat Ashkenazi (3) |
5,770 | 320,004 | 325,774 | |||||||||
| Sean Cunningham |
4,616 | — | 4,616 | |||||||||
| Benjamin Daverman |
6,058 | — | 6,058 | |||||||||
| Susannah Gray (3) |
6,924 | 320,004 | 326,928 | |||||||||
| Robert B. Hance (4) |
4,616 | 320,004 | 324,620 | |||||||||
| Jessica Hopfield (3) |
7,212 | 320,004 | 327,216 | |||||||||
| Gregory T. Lucier (3) |
4,616 | 320,004 | 324,620 | |||||||||
| Luke Marker |
4,616 | — | 4,616 | |||||||||
| Constantine Mihas |
7,500 | — | 7,500 | |||||||||
| Murali K. Prahalad (5) |
4,616 | 320,004 | 324,620 | |||||||||
| (1) | The amounts in this column represent the portion of quarterly fees paid in January 2021 attributable to board service from November 19, 2020 through December 31, 2020. |
| (2) | The amounts in this column reflect the grant date fair value of restricted stock units granted in connection with our IPO, as computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, Compensation – Stock Compensation. The assumptions used in calculating the grant date fair value of the restricted stock units with respect to our Class A common stock reported in the Stock Awards column are set forth in Note 10 to the consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2020, incorporated herein by reference. These awards will vest annually over three years from the grant date subject to the recipients continuous service on the Board through each vesting date. The members of our Board who are employees of GTCR or its affiliates are not eligible to receive stock awards in connection with their service as members of our Board. |
| (3) | As of December 31, 2020, each of Mr. Ashkenazi, Ms. Gray, Ms. Hopfield, and Mr. Lucier held 11,852 unvested restricted stock units with respect to our Class A common stock, which will vest in equal installments on each of November 19, 2021, 2022 and 2023, subject to such director’s continued service. |
| (4) | As of December 31, 2020, Mr. Hance held (i) 6,000 vested incentive units and 4,000 unvested incentive units (which will vest in equal installments, subject to Mr. Hance’s continued service, on January 1 of 2021 and 2022) in MLSH 1 and (ii) 11,852 unvested restricted stock units with respect to our Class A common stock, which will vest in equal installments on each of November 19, 2021, 2022 and 2023, subject to Mr. Hance’s continued service. |
| (5) | As of December 31, 2020, Dr. Prahalad held 8,000 vested incentive units and 2,000 unvested incentive units (which will vest, subject to Dr. Prahalad’s continued service, on August 10, 2021) in MLSH 1 and (ii) 11,852 unvested restricted stock units with respect to our Class A common stock, which will vest in equal installments on each of November 19, 2021, 2022 and 2023 subject to Mr. Prahalad’s continued service. |
37
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
PRINCIPAL AND SELLING STOCKHOLDERS
The following table sets forth information about the beneficial ownership of our Class A common stock and Class B common stock as of December 31, 2020, and as adjusted to reflect the sale of the Class A common stock offered by the selling stockholders in this offering for:
| • | each person or group known to us who beneficially owns more than 5% of our Class A common stock or Class B common stock immediately prior to this offering; |
| • | each of our directors; |
| • | each of our Named Executive Officers; |
| • | each of the selling stockholders; |
| • | all of our directors and executive officers as a group. |
Each stockholder’s percentage ownership before the offering is based on the numbers of shares of Class A common stock and Class B common stock (together with the same amount of LLC Units) outstanding as of December 31, 2020. The selling stockholders have granted the underwriters an option to purchase up to additional shares of Class A common stock.
The numbers of shares of Class A common stock and Class B common stock (together with the same amount of LLC Units) beneficially owned and percentages of beneficial ownership after the offering that are set forth below are based on shares of Class A common stock to be issued and outstanding immediately after the offering, assuming no exercise by the underwriters of their option to purchase additional shares, and shares, assuming exercise by the underwriters of their option to purchase additional shares..
Unless otherwise noted below, the address for each beneficial owner listed on the table is 10770 Wateridge Circle Suite 200, San Diego, California, 92121. We have determined beneficial ownership in accordance with the rules of the SEC. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the tables below have sole voting and investment power with respect to all Class A common stock that they beneficially own, subject to applicable community property laws.
| Shares of Common Stock Beneficially Owned Prior to this Offering |
Shares of Common Stock Beneficially Owned After this Offering |
|||||||||||||||||||||||||||||||||||||||
| Name of Beneficial |
Shares of Class A Common Stock |
% of Class A Common Stock Outstanding |
Shares of Class B Common Stock |
% of Class B Common Stock Outstanding |
% of Combined Voting Power(1) |
Number of Class A Shares Being Offered |
Shares of Class A Common Stock |
Shares of Class B Common Stock |
% of Combined Voting Power Assuming the Underwriters’ Option Is Not Exercised(1) |
% of Combined Voting Power Assuming the Underwriters’ Option Is Exercised in Full(1) |
||||||||||||||||||||||||||||||
| 5% Stockholders: |
||||||||||||||||||||||||||||||||||||||||
| GTCR(2) |
||||||||||||||||||||||||||||||||||||||||
| FMR LLC(3) |
||||||||||||||||||||||||||||||||||||||||
| Vanguard Group Inc.(4) |
||||||||||||||||||||||||||||||||||||||||
| D1 Capital Partners L.P.(5) |
||||||||||||||||||||||||||||||||||||||||
| Named Executive Officers and Directors: |
||||||||||||||||||||||||||||||||||||||||
| Carl Hull |
||||||||||||||||||||||||||||||||||||||||
| Kevin Herde |
||||||||||||||||||||||||||||||||||||||||
| Brian Neel |
||||||||||||||||||||||||||||||||||||||||
| Anat Ashkenazi |
||||||||||||||||||||||||||||||||||||||||
| Sean Cunningham |
||||||||||||||||||||||||||||||||||||||||
| Benjamin Daverman |
||||||||||||||||||||||||||||||||||||||||
| Susannah Gray |
||||||||||||||||||||||||||||||||||||||||
| Robert B. Hance |
||||||||||||||||||||||||||||||||||||||||
| Jessica Hopfield |
||||||||||||||||||||||||||||||||||||||||
| Gregory T. Lucier |
||||||||||||||||||||||||||||||||||||||||
| Luke Marker |
||||||||||||||||||||||||||||||||||||||||
| Constantine Mihas |
||||||||||||||||||||||||||||||||||||||||
| Murali K. Prahalad |
||||||||||||||||||||||||||||||||||||||||
| All executive officers and directors as a group (17 individuals) |
||||||||||||||||||||||||||||||||||||||||
38
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| (1) | Each share of Class A common stock and Class B common stock entitles the registered holder thereof to one vote for each share on all matters presented to stockholders for a vote generally, including the election of directors. The Class A common stock and Class B common stock vote as a single class on all matters except as required by law or the certificate of incorporation. |
| (2) | Represents shares of Class A common stock held directly by MLSH 2 and shares of Class B common stock held directly by MLSH 1. MLSH 1 and MLSH 2 are each managed by a board of managers. GTCR Fund XI/C LP controls the board of managers of MLSH 2. GTCR Fund XI/B LP and GTCR Co-Invest XI LP control the board of managers of MLSH 1. This number excludes shares of Class A common stock issuable in exchange for LLC Units held by MLSH 1. These shares of Class A common stock represent approximately % of the shares of Class A common stock that would be outstanding immediately after this offering if all outstanding LLC Units were exchanged and all outstanding shares of Class B common stock were converted at that time. GTCR Partners XI/A&C LP is the general partner of GTCR Fund XI/C LP. GTCR Partners XI/B LP is the general partner of GTCR Fund XI/B LP. GTCR Investment XI LLC is the general partner of each of GTCR Co-Invest XI LP, GTCR Partners XI/A&C LP and GTCR Partners XI/B LP. GTCR Investment XI LLC is managed by a board of managers (the “GTCR Board of Managers”) consisting of Mark M. Anderson, Craig A. Bondy, Aaron D. Cohen, Sean L. Cunningham, Benjamin J. Daverman, David A. Donnini, Constantine S. Mihas and Collin E. Roche, and no single person has voting or dispositive authority over the Class A common stock or Class B common stock. Each of GTCR Partners XI/A&C LP, GTCR Investment XI LLC and the GTCR Board of Managers may be deemed to share beneficial ownership of the shares held of record by MLSH 2, each of GTCR Partners XI/B LP, GTCR Investment XI LLC and the GTCR Board of Managers may be deemed to share beneficial ownership of the shares held of record by MLSH 1 and each of the individual members of the GTCR Board of Managers disclaims beneficial ownership of the shares held of record by MLSH 1 and MLSH 2 except to the extent of his pecuniary interest therein. The address for each of MLSH 1, MLSH 2, GTCR Fund XI/C LP, GTCR Fund XI/B LP, GTCR Co-Invest XI LP, GTCR Partners XI/A&C LP, GTCR Partners XI/B LP and GTCR Investment XI LLC is 300 North LaSalle Street, Suite 5600, Chicago, IL, 60654, and their telephone number is (312) 382-2200. |
| (3) | Based upon information reported by way of a Schedule 13G filed by FMR LLC with the SEC on February 8, 2021. Represents shares of Class A common stock held directly by FMR LLC and Abigail P. Johnson. Abigail P. Johnson is a Director, the Chairman and the Chief Executive Officer of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act (“Fidelity Funds”) advised by Fidelity Management & Research Company LLC (“FMR Co. LLC”), a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees. FMR Co. LLC carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. This footnote reflects the securities beneficially owned, or that may be deemed to be beneficially owned, by FMR LLC, certain of its subsidiaries and affiliates, and other companies (collectively, the “FMR Reporters”). This footnote does not reflect securities, if any, beneficially owned by certain other companies whose beneficial ownership of securities is disaggregated from that of the FMR Reporters in accordance with Securities and Exchange Commission Release No. 34-39538 (January 12, 1998). The address for FMR LLC is 245 Summer Street, Boston, MA 02210 and its telephone number is (617) 570-6339. The address for Abigail P. Johnson is 245 Summer Street, Boston, MA 02210 and her telephone number is (617) 570-6339. |
| (4) | Based upon information reported by way of a Schedule 13G filed by The Vanguard Group with the SEC on February 10, 2021. Represents shares of Class A common stock beneficially owned by the following subsidiaries of The Vanguard Group: Vanguard Asset Management, Limited, Vanguard Fiduciary Trust Company, Vanguard Global Advisors, LLC, Vanguard Group (Ireland) Limited, Vanguard Investments Australia Ltd, Vanguard Investments Canada Inc., Vanguard Investments Hong Kong Limited, and Vanguard Investments UK, Limited. The Vanguard Group has sole voting power, shared voting power, sole dispositive power and shared dispositive power over , , and shares of Class A common stock, respectively, listed in the table above. The address for The Vanguard Group is 100 Vanguard Blvd., Malvern, PA 19355 and its telephone number is (610) 669-1000. |
| (5) | Based upon information reported by way of a Schedule 13G filed by D1 Capital Partners L.P. (the “Investment Manager”) with the SEC on February 16, 2021. Represents shares of Class A common stock held directly by the Investment Manager and Mr. Daniel Sundheim. The Investment Manager is a registered investment adviser and serves as the investment manager of private investment vehicles and accounts, including D1 Capital Partners Master LP (the “Investment Vehicle”). Mr. Sundheim may be deemed to beneficially own the reported securities by virtue of the fact that Mr. Sundheim indirectly controls the Investment Manager. The Investment Manager and Mr. Sundheim have shared voting power and shared dispositive power over shares of Class A common stock listed in the table above. The address for the Investment Manager and Mr. Sundheim is 9 West 57th Street, 36th Floor, New York, NY 10019 and their telephone number is (212) 390-9100. |
39
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Policies for Approval of Related Party Transactions
In connection with our IPO, we adopted a policy with respect to the review, approval and ratification of related party transactions. Under the policy, our Audit Committee is responsible for reviewing and approving related party transactions. In the course of its review and approval of related party transactions, our Audit Committee considers the relevant facts and circumstances to decide whether to approve such transactions. In particular, our policy requires our Audit Committee to consider, among other factors it deems appropriate:
| • | the related person’s relationship to us and interest in the transaction; |
| • | the material facts of the proposed transaction, including the proposed aggregate value of the transaction; |
| • | the impact on a director or a director nominee’s independence in the event the related person is a director or an immediate family member of the director or director nominee; |
| • | the benefits to us of the proposed transaction; |
| • | if applicable, the availability of other sources of comparable products or services; and |
| • | an assessment of whether the proposed transaction is on terms that are comparable to the terms available to an unrelated third party or to employees generally. |
The Audit Committee may only approve those transactions that are in, or are not inconsistent with, our best interests and those of our stockholders, as the Audit Committee determines in good faith.
Amended and Restated Operating Agreement
In connection with our IPO, we amended and restated Topco LLC’s existing operating agreement, which we refer to as the “LLC Operating Agreement.” The operations of Topco LLC and the rights and obligations of the LLC Unitholders are set forth in the LLC Operating Agreement.
Registration Rights Agreement
In connection with our IPO, we entered into a registration rights agreement with MLSH 1 and MLSH 2. MLSH 1 and MLSH 2 are entitled to request that we register their shares of capital stock on a long-form or short-form registration statement on one or more occasions in the future, which registrations may be “shelf registrations.” MLSH 1 and MLSH 2 are entitled to participate in certain of our registered offerings, subject to the restrictions in the registration rights agreement. We will pay expenses in connection with the exercise of these rights. The registration rights described in this paragraph apply to (1) shares of our Class A common stock held by MLSH 1 and MLSH 2 and their affiliates, and (2) any of our capital stock (or that of our subsidiaries) issued or issuable with respect to the Class A common stock described in clause (1) with respect to any dividend, distribution, recapitalization, reorganization, or certain other corporate transactions (“Registrable Securities”). These registration rights are also for the benefit of any subsequent holder of Registrable Securities; provided that any particular securities will cease to be Registrable Securities when they have been sold in a registered public offering, sold in compliance with Rule 144 of the Securities Act or repurchased by us or our subsidiaries. In addition, with the consent of the company and holders of a majority of Registrable Securities, certain Registrable Securities will cease to be Registrable Securities if they can be sold without limitation under Rule 144 of the Securities Act.
Tax Receivable Agreement
In connection with our IPO, we entered into a Tax Receivable Agreement with MLSH 1 and MLSH 2 that provides for the payment from time to time by us to MLSH 1 and MLSH 2, collectively, of 85% of the amount of
40
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
the benefits, if any, that we realize or, under certain circumstances, are deemed to realize as a result of (i) certain increases in the tax basis of assets of Topco LLC and its subsidiaries resulting from purchases or exchanges of LLC Units, (ii) certain tax attributes of the Blocker Entities, Topco LLC and subsidiaries of Topco LLC that existed prior to the IPO and (iii) certain other tax benefits related to our entering into the Tax Receivable Agreement, including tax benefits attributable to payments that we make under the Tax Receivable Agreement.
Director Nomination Agreement
In connection with our IPO, we entered into a Director Nomination Agreement with GTCR. The Director Nomination Agreement provides GTCR the right to nominate to the Board a number of designees equal to at least: (i) 100% of the total number of directors comprising the Board, so long as GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 40% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO, (ii) 40% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 30% but less than 40% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO, (iii) 30% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 20% but less than 30% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO, (iv) 20% of the total number of directors, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 10% but less than 20% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO and (v) one director, in the event that GTCR beneficially owns shares of Class A common stock and Class B common stock representing at least 5% of the total amount of shares of Class A common stock and Class B common stock it owned as of the date of the IPO. In each case, GTCR’s nominees must comply with applicable law and stock exchange rules. In addition, GTCR shall be entitled to designate the replacement for any of its Board designees whose Board service terminates prior to the end of the director’s term, regardless of GTCR’s beneficial ownership at that time. GTCR shall also have the right to have its designees participate on committees of our Board proportionate to its voting power, subject to compliance with applicable law and stock exchange rules. The Director Nomination Agreement also prohibits us from increasing or decreasing the size of our Board without the prior written consent of GTCR. This agreement will terminate at such time as GTCR beneficially owns less than 5% of the shares of Class A and Class B common stock it beneficially owned as of the date of the IPO.
Indemnification of Officers and Directors
We entered into indemnification agreements with each of our officers and directors. The indemnification agreements provide the officers and directors with contractual rights to indemnification, expense advancement and reimbursement, to the fullest extent permitted under Delaware law. Additionally, we may enter into indemnification agreements with any new directors or officers that may be broader in scope than the specific indemnification provisions contained in Delaware law. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our officers and directors pursuant to the foregoing agreements, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is therefore unenforceable.
Relationship with GTCR
We previously utilized GTCR, who controls the vote of all matters submitted to a vote of our stockholders, for certain services pursuant to an advisory services agreement. Under the agreement, GTCR provided us with financial and management consulting services in the areas of corporate strategy, budgeting for future corporate investments, acquisition and divestiture strategies and debt and equity financings. We paid a $0.1 million quarterly management fee to GTCR for these services. We also reimbursed GTCR for out-of-pocket expenses
41
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
incurred while providing these services. The advisory services agreement also required that we pay placement fees to GTCR of 1.0% of the gross amount of any debt or equity financings, including the IPO. The advisory services agreement terminated in connection with the IPO.
We paid GTCR $4.2 million, $0.5 million, and $0.5 million in each of the years ended December 31, 2020, 2019, and 2018, respectively, for services in connection with the advisory services agreement. We may continue to engage GTCR from time to time, subject to compliance with our related party transactions policy.
During the year ended December 31, 2018, $52.0 million of capital distributions were made to certain legacy unit holders of MLSC Holdings, LLC (“MLSC”), the parent entity of Cygnus, including GTCR. The 2018 distribution was treated as a preferred return of capital per the terms of the MLSC limited liability company agreement. There were no such distributions made during the year ended December 31, 2019. A tax distribution of $0.3 million was made to the non-controlling interest holders of MLSC during the year ended December 31, 2020.
In October 2020, we paid GTCR a placement fee of $3.7 million in connection with our entry into the New Credit Agreement.
Lease Arrangements
Cygnus Technologies, a subsidiary of Topco LLC., has an ongoing lease agreement for facilities in Southport, NC with an entity controlled by a close relative of the president of Cygnus Technologies. The close relative was also previously an employee of Cygnus Technologies who terminated their employment during the year ended December 31, 2018. The president of Cygnus Technologies also personally financed a loan to this entity, which was used to acquire the property leased by Cygnus Technologies. The lease terms are considered to be consistent with market rates.
Cygnus Technologies paid $0.2 million of rent under this lease agreement for the years ended December 31, 2020, 2019 and 2018, respectively.
Noncontrolling Interests
The noncontrolling interests in MLSC Holdings, LLC, the parent of Cygnus Technologies, represents equity interest that was retained by the unit holders of the MLSC Holdings, LLC entity prior to its acquisition by Maravai. The president of Cygnus Technologies and his affiliated entity are the holders of the noncontrolling interests.
42
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
The following is a description of the material terms of our amended and restated certificate of incorporation and amended and restated bylaws. The following description may not contain all of the information that is important to you. To understand the material terms of our Class A common stock, you should read our amended and restated certificate of incorporation (our “certificate”) and amended and restated bylaws (our “bylaws”), copies of which are filed with the SEC as exhibits to the registration statement, of which this prospectus is a part.
General
As of December 31, 2020, our certificate authorized capital stock consisting of:
| • | 500,000,000 shares of Class A common stock, par value $0.01 per share; |
| • | 300,000,000 shares of Class B common stock, par value $0.01 per share; and |
| • | 50,000,000 shares of preferred stock, with a par value per share that may be established by the Board in the applicable certificate of designations. |
The selling stockholders are selling shares of Class A common stock in this offering ( shares if the underwriters exercise in full their option to purchase additional shares). As of December 31, 2020, we had shares of Class A common stock and shares of Class B common stock outstanding, respectively, and no shares of preferred stock outstanding. Further, shares of Class A common stock are issuable upon the exercise of outstanding stock options and shares of Class A common stock are issuable upon the vesting and settlement of outstanding RSUs. Upon completion of this offering, we expect to have shares of Class A common stock outstanding ( shares if the underwriters exercise in full their option to purchase additional shares) and shares of Class B common stock outstanding.
The following summary describes the material provisions of our capital stock and is qualified in its entirety by reference to the certificate and our bylaws and to the applicable provisions of the DGCL. We urge you to read our certificate and our bylaws, which are included as exhibits to the registration statement of which this prospectus forms a part.
Certain provisions of our certificate and our bylaws summarized below may be deemed to have an anti-takeover effect and may delay or prevent a tender offer or takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares of common stock.
Class A Common Stock
Holders of shares of our Class A common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. The holders of our Class A common stock do not have cumulative voting rights in the election of directors.
Holders of shares of our Class A common stock vote together with holders of our Class B common stock as a single class on all matters presented to our stockholders for their vote or approval, except for certain amendments to our certificate of incorporation described below or as otherwise required by applicable law or the certificate.
Holders of shares of our Class A common stock are entitled to receive dividends when and if declared by our Board out of funds legally available therefor, subject to any statutory or contractual restrictions on the payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock.
43
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Upon our dissolution or liquidation or the sale of all or substantially all of our assets, after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of shares of our Class A common stock are entitled to receive pro rata our remaining assets available for distribution.
Holders of shares of our Class A common stock do not have preemptive, subscription, redemption or conversion rights. There are no redemption or sinking fund provisions applicable to the Class A common stock.
Class B Common Stock
Holders of shares of our Class B common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. The holders of our Class B common stock do not have cumulative voting rights in the election of directors.
Holders of shares of our Class B common stock vote together with holders of our Class A common stock as a single class on all matters presented to our stockholders for their vote or approval, except for certain amendments to our certificate of incorporation described below or as otherwise required by applicable law or the certificate.
Holders of our Class B common stock do not have any right to receive dividends or to receive a distribution upon dissolution or liquidation or the sale of all or substantially all of our assets. Additionally, holders of shares of our Class B common stock do not have preemptive, subscription, redemption or conversion rights. There are no redemption or sinking fund provisions applicable to the Class B common stock. Any amendment of our certificate of incorporation that gives holders of our Class B common stock (1) any rights to receive dividends or any other kind of distribution, (2) any right to convert into or be exchanged for Class A common stock or (3) any other economic rights will require, in addition to stockholder approval, the affirmative vote of holders of our Class A common stock voting separately as a class.
MLSH 1 owns 100% of our outstanding Class B common stock.
Preferred Stock
We have no shares of preferred stock outstanding.
Under the terms of our certificate, our Board is authorized to direct us to issue shares of preferred stock in one or more series without stockholder approval. Our Board has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our Board to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock. Additionally, the issuance of preferred stock may adversely affect the holders of our Class A common stock by restricting dividends on the Class A common stock, diluting the voting power of the Class A common stock or subordinating the liquidation rights of the Class A common stock. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of our Class A common stock.
44
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Forum Selection
Our certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any state court action for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, (3) any action asserting a claim against Maravai LifeSciences Holdings, Inc. or any director or officer thereof arising pursuant to any provision of the DGCL or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware, our certificate of incorporation or our bylaws or (4) any other action asserting a claim against Maravai LifeSciences Holdings, Inc. or any director or officer thereof that is governed by the internal affairs doctrine; provided that, for the avoidance of doubt, the forum selection provision that identifies the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation, including any “derivative action,” will not apply to suits to enforce a duty or liability created by the Securities Act, the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our certificate of incorporation also provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock will be deemed to have notice of and to have consented to the provisions of our certificate of incorporation described above. Although we believe these provisions benefit us by providing increased consistency in the application of Delaware law or the Securities Act, as applicable, for the specified types of actions and proceedings, the provisions may have the effect of discouraging lawsuits against us or our directors and officers. Alternatively, if a court were to find any of the forum selection provisions contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable, we may incur additional costs associated with having to litigate such action in other jurisdictions, which could have an adverse effect on our business, financial condition, results of operations, cash flows and prospects and result in a diversion of the time and resources of our employees, management and board of directors.
Anti-Takeover Provisions
Our certificate, bylaws and the DGCL contain provisions, which are summarized in the following paragraphs, that are intended to enhance the likelihood of continuity and stability in the composition of our Board. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile change of control and enhance the ability of our Board to maximize stockholder value in connection with any unsolicited offer to acquire us. However, these provisions may have an anti-takeover effect and may delay, deter or prevent a merger or acquisition of us by means of a tender offer, a proxy contest or other takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the prevailing market price for the shares of Class A common stock held by stockholders.
These provisions include:
Classified Board. Our certificate provides that our Board is divided into three classes of directors, with the classes as nearly equal in number as possible, and with the directors serving three-year terms. As a result, approximately one-third of our Board is elected each year. The classification of the directors has the effect of making it more difficult for stockholders to change the composition of our Board. Our certificate also provides that, subject to any rights of holders of preferred stock to elect additional directors under specified circumstances, the number of directors will be fixed exclusively pursuant to a resolution adopted by our Board. Our Board currently has 11 members.
Stockholder Action by Written Consent. Our certificate precludes stockholder action by written consent at any time when GTCR controls, in the aggregate, less than 35% in voting power of our outstanding common stock.
45
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Special Meetings of Stockholders. Our certificate and bylaws provides that, except as required by law, special meetings of our stockholders may be called at any time only by or at the direction of our Board or the chairman of our Board; provided, however, at any time when GTCR controls, in the aggregate, at least 35% in voting power of our outstanding common stock, special meetings of our stockholders shall also be called by our Board or the chairman of our Board at the request of GTCR. Our bylaws prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in control or management of us.
Advance Notice Procedures. Our bylaws establish advance notice procedures for stockholder proposals and nomination of candidates for election as directors, other than nominations made by or at the direction of our Board or a committee of our Board, and provided, however, that at any time when GTCR controls, in the aggregate, at least 10% of the voting power of our outstanding common stock, such advance notice procedure does not apply to GTCR. Stockholders at an annual meeting will only be able to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our Board or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given our Secretary timely written notice, in proper form, of the stockholder’s intention to bring that business before the meeting. Although the bylaws do not give our Board the power to approve or disapprove stockholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting, the bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of us. These provisions do not apply to nominations by GTCR pursuant to the Director Nomination Agreement. See “Certain Relationships and Related Party Transactions—Director Nomination Agreement” for more details with respect to the Director Nomination Agreement.
Removal of Directors; Vacancies. Our certificate provides that a director nominated by GTCR may be removed with or without cause by GTCR; provided, however, that at any time when GTCR controls less than 40% in voting power of our outstanding common stock, all directors, including those nominated by GTCR, may only be removed for cause, and only by the affirmative vote of holders of at least 66 2/3% in voting power of all the then-outstanding shares of capital stock of the company entitled to vote thereon, voting together as a single class. In addition, our certificate also provides that, subject to the rights granted to one or more series of preferred stock then outstanding, any newly created directorship on our Board that results from an increase in the number of directors and any vacancies on our Board will be filled only by the affirmative vote of a majority of the remaining directors, even if less than a quorum, or by a sole remaining director (and not by the stockholders).
Supermajority Approval Requirements. Our certificate and bylaws provide that our Board is expressly authorized to make, alter, amend, change, add to, rescind or repeal, in whole or in part, our bylaws without a stockholder vote in any matter not inconsistent with the laws of the State of Delaware and our certificate. For as long as GTCR controls, in the aggregate, at least 50% in voting power of our outstanding common stock, any amendment, alteration, rescission or repeal of our bylaws by our stockholders requires the affirmative vote of a majority in voting power of the outstanding shares of our stock entitled to vote on such amendment, alteration, change, addition, rescission or repeal. At any time when GTCR controls, in the aggregate, less than 50% in voting power of our outstanding common stock, any amendment, alteration, rescission or repeal of our bylaws by our stockholders requires the affirmative vote of the holders of at least 66 2/3% in voting power of all the then-outstanding shares of stock of the company entitled to vote thereon, voting together as a single class.
The DGCL provides generally that the affirmative vote of a majority of the outstanding shares entitled to vote thereon, voting together as a single class, is required to amend a corporation’s certificate of incorporation, unless the certificate requires a greater percentage.
46
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Our certificate provides that the following provisions in our certificate may be amended, altered, repealed or rescinded only by the affirmative vote of the holders of at least 66 2/3% (as opposed to a majority threshold) in voting power of all the then-outstanding shares of stock entitled to vote thereon, voting together as a single class:
| • | the provision requiring a 66 2/3% supermajority vote for stockholders to amend our bylaws; |
| • | the provisions providing for a classified board of directors (the election and term of our directors); |
| • | the provisions regarding resignation and removal of directors; |
| • | the provisions regarding entering into business combinations with interested stockholders; |
| • | the provisions regarding stockholder action by written consent; |
| • | the provisions regarding calling special meetings of stockholders; |
| • | the provisions regarding filling vacancies on our Board and newly created directorships; |
| • | the provision establishing the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation; |
| • | the provision establishing the federal district courts of the United States as the exclusive forum for litigation arising under the Securities Act; |
| • | the provisions eliminating monetary damages for breaches of fiduciary duty by a director; and |
| • | the amendment provision requiring that the above provisions be amended only with a 66 2/3% supermajority vote. |
The combination of the classification of our Board, the lack of cumulative voting and the supermajority voting requirements will make it more difficult for our existing stockholders to replace our Board as well as for another party to obtain control of us by replacing our Board. Because our Board has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management.
Authorized but Unissued Shares. Our authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to stock exchange rules. These additional shares of capital stock may be utilized for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. One of the effects of the existence of authorized but unissued common stock or preferred stock may be to enable our Board to issue shares of capital stock to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of the company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our stockholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Business Combinations. We are not subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that the person becomes an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15% or more of the corporation’s voting stock.
Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions: (1) before the stockholder became an interested stockholder, the Board approved either the business combination or the transaction which resulted in the
47
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
stockholder becoming an interested stockholder; (2) upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances; or (3) at or after the time the stockholder became an interested stockholder, the business combination was approved by the Board and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares.
We have opted out of Section 203; however, our certificate contains similar provisions providing that we may not engage in certain “business combinations” with any “interested stockholder” for a three-year period following the time that the stockholder became an interested stockholder, unless:
| • | prior to such time, our Board approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding certain shares; or |
| • | at or subsequent to that time, the business combination is approved by our Board and by the affirmative vote of holders of at least 66 2/3% of our outstanding voting stock that is not owned by the interested stockholder. |
Under certain circumstances, this provision will make it more difficult for a person who would be an “interested stockholder” to effect various business combinations with us for a three-year period. This provision may encourage companies interested in acquiring us to negotiate in advance with our Board because the stockholder approval requirement would be avoided if our Board approves either the business combination or the transaction which results in the stockholder becoming an interested stockholder. These provisions also may have the effect of preventing changes in our Board and may make it more difficult to accomplish transactions which stockholders may otherwise deem to be in their best interests.
Our certificate of incorporation provides that GTCR, and any of its direct or indirect transferees and any group as to which such persons are a party, do not constitute “interested stockholders” for purposes of this provision.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. Our certificate includes a provision that eliminates the personal liability of directors for monetary damages for any breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions is to eliminate the rights of us and our stockholders, through stockholders’ derivative suits on our behalf, to recover monetary damages from a director for breach of fiduciary duty as a director, including breaches resulting from grossly negligent behavior. However, exculpation will not apply to any director if the director has acted in bad faith, knowingly or intentionally violated the law, authorized illegal dividends or redemptions or derived an improper benefit from his or her actions as a director.
48
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Our bylaws provide that we must indemnify and advance expenses to our directors and officers to the fullest extent authorized by the DGCL. We are also expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers and certain employees for some liabilities. We believe that these indemnification and advancement provisions and insurance are useful to attract and retain qualified directors and officers.
The limitation of liability, indemnification and advancement provisions may discourage stockholders from bringing a lawsuit against directors for breaches of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought.
Corporate Opportunity Doctrine
Delaware law permits corporations to adopt provisions renouncing any interest or expectancy in certain opportunities that are presented to the corporation or its officers, directors or stockholders. Our certificate, to the maximum extent permitted from time to time by Delaware law, renounces any interest or expectancy that we have in, or right to be offered an opportunity to participate in, specified business opportunities that are from time to time presented to certain of our officers, directors or stockholders or their respective affiliates, other than those officers, directors, stockholders or affiliates who are our or our subsidiaries’ employees. Our certificate provides that, to the fullest extent permitted by law, none of GTCR or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his or her director and officer capacities) or its, his or her affiliates has any duty to refrain from (1) engaging in a corporate opportunity in the same or similar lines of business in which we or our affiliates now engage or propose to engage or (2) otherwise competing with us or our affiliates. In addition, to the fullest extent permitted by law, in the event that GTCR or any non-employee director acquires knowledge of a potential transaction or other business opportunity which may be a corporate opportunity for itself, himself or herself or its, his or her affiliates or for us or our affiliates, such person will have no duty to communicate or offer such transaction or business opportunity to us or any of our affiliates and they may take any such opportunity for themselves or offer it to another person or entity. Our certificate does not renounce our interest in any business opportunity that is expressly offered to a non-employee director solely in his or her capacity as a director or officer of Maravai LifeSciences Holdings, Inc. To the fullest extent permitted by law, no business opportunity will be deemed to be a potential corporate opportunity for us unless we would be permitted to undertake the opportunity under our certificate, we have sufficient financial resources to undertake the opportunity and the opportunity would be in line with our business.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, our stockholders have appraisal rights in connection with a merger or consolidation of Maravai LifeSciences Holdings, Inc. Pursuant to the DGCL, stockholders who properly request and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares of capital stock as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of our stockholders may bring an action in our name to procure a judgment in our favor, also known as a derivative action, provided that the stockholder bringing the action is a holder of our shares of capital stock at the time of the transaction to which the action relates or such stockholder’s stock thereafter devolved by operation of law.
49
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Transfer Agent and Registrar
The transfer agent and registrar for our Class A common stock is American Stock Transfer & Trust Company, LLC. Its address is 6201 15th Avenue, Brooklyn, New York, 11219.
Listing
Our Class A common stock is listed on The Nasdaq Global Select Market under the trading symbol “MRVI.”
50
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of our Class A common stock in the public market, or the perception that such sales may occur, could adversely affect the prevailing market price of our Class A common stock. No prediction can be made as to the effect, if any, future sales of shares, or the availability of shares for future sales, will have on the market price of our common stock prevailing from time to time. We also cannot predict with certainty when or if GTCR will otherwise sell its Class A common stock. The sale of substantial amounts of our Class A common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of our common stock.
As of December 31, 2020, shares of our Class A common stock were outstanding. Of these shares, shares sold in our prior public offering and the shares to be sold in this offering will be freely tradable without restriction or further registration under the Securities Act, except that any shares held by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, would only be able to be sold in compliance with the conditions of Rule 144.
Sale of Restricted Shares
The remaining shares of Class A common stock (or shares of Class A common stock, including shares of Class A common stock issuable upon redemption or exchange of the LLC Units, as described below) will be “restricted securities,” as that phrase is defined in Rule 144, and may be resold only after registration under the Securities Act or pursuant to an exemption from such registration, including, among others, the exemptions provided by Rule 144 and 701 under the Securities Act, which rules are summarized below. These remaining shares of Class A common stock that will be outstanding upon completion of this offering will be available for sale in the public market after the expiration of market stand-off agreements with us and the lock-up agreements described in “Underwriters,” taking into account the provisions of Rules 144 and 701 under the Securities Act.
In addition, pursuant to the Exchange Agreement, MLSH 1 may from time to time exchange its LLC Units for shares of Class A common stock on a one-for-one basis, or, at our election, for cash, from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). MLSH 1 is also required to deliver to us a number of shares of Class B common stock equivalent to the number of shares of Class A common stock being exchanged to effectuate an exchange. Any shares of Class B common stock so delivered will be cancelled. MLSH 1 holds LLC Units, all of which will be exchangeable for shares of our Class A common stock or, at our election, for cash from a substantially concurrent public offering or private sale (based on the price of our Class A common stock in such public offering or private sale). The shares of Class A common stock we issue upon such exchanges would be “restricted securities” as defined in Rule 144 unless we register such issuances. However, we entered into a registration rights agreement with MLSH 1 that requires us to register these shares of Class A common stock, subject to certain conditions. See “—Registration Rights” and “Certain Relationships and Related Party Transactions—Registration Rights Agreement.”
Under the terms of the LLC Operating Agreement, except pursuant to a valid exchange under the terms of the Exchange Agreement, all of the LLC Units held by MLSH 1 are subject to restrictions on disposition.
Rule 144
In general, under Rule 144, any person who is not our affiliate, who was not our affiliate at any time during the preceding three months and who has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, subject to the availability of current public information about us and subject to applicable lock-up restrictions. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior
51
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
owner other than one of our affiliates, then that person would be entitled to sell those shares without complying with any of the requirements of Rule 144, subject to applicable lock-up restrictions.
Subject to applicable lock-up restrictions, a person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of shares within any three month period that does not exceed the greater of: (1) 1% of the number of shares of our Class A common stock outstanding, which will equal approximately shares immediately after this offering (or shares if the underwriters exercise in full their option to purchase additional shares); and (2) the average weekly trading volume of our common stock on the NASDAQ during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions, notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701, any of our employees, directors, officers, consultants or advisors who acquired shares of capital stock from us in connection with a compensatory stock or option plan or other compensatory written agreement before the effective date of the IPO are, subject to applicable lock-up restrictions, eligible to resell such shares in reliance upon Rule 144 beginning 90 days after the date of our IPO. If such person is not an affiliate and was not our affiliate at any time during the preceding three months, the sale may be made subject only to the manner-of-sale restrictions of Rule 144. If such a person is an affiliate, the sale may be made under Rule 144 without compliance with holding period requirements under Rule 144, but subject to the other Rule 144 restrictions described above.
Stock Plans
We have filed a registration statement on Form S-8 under the Securities Act to register shares of our Class A common stock issued or reserved for issuance under the 2020 Plan. Accordingly, shares of Class A common stock registered under such registration statement are generally eligible available for resale in the open market following the effective date, unless such shares are subject to vesting restrictions with us, Rule 144 restrictions applicable to our affiliates or the lock-up restrictions described below.
Lock-Up Agreements
Our directors, our executive officers and holders of substantially all of our capital stock and securities convertible into our capital stock entered into lock-up agreements with the underwriters in connection with the IPO pursuant to which each of these persons or entities, with limited exceptions, for a lock-up period ending May 18, 2021, may not, without the prior written consent of representatives of such underwriters, (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, including LLC Units (“Lock-Up Securities”); (2) file or confidentially submit any registration statement with the SEC relating to the offering of any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; or (3) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any Lock-Up Securities. Upon the expiration of the lock-up period, substantially all of the shares subject to such lock-up restrictions will become eligible for sale, subject to the limitations discussed above.
In connection with this offering, Morgan Stanley & Co. LLC, and , as representatives of the several underwriters of our IPO, have agreed to release the restrictions under the lock-up agreements that
52
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
were executed in connection with our IPO with respect to up to shares of our Class A common stock to be sold in this offering that are held by the selling stockholders, which includes shares beneficially owned by our directors and executive officers or entities with which they are affiliated; provided, however, that the release of shares of our common stock held by the selling stockholders is limited to the shares actually sold in this offering. Morgan Stanley & Co. LLC, and , as representatives of the several underwriters of our IPO, may, in their sole discretion, from time to time permit our stockholders to sell additional shares and waive the contractual lock-up prior to the expiration of the lock-up agreements.
In addition, subject to certain exceptions described in the section titled “Underwriters,” our directors and executive officers and GTCR have entered into lock-up agreements with the underwriters of this offering pursuant to which they have agreed that subject to certain exceptions, they will not directly or indirectly sell or dispose of any shares of Class A common stock or any securities convertible into or exchangeable or exercisable for shares of Class A common stock for a period of 90 days after the date of this prospectus. The lock-up restrictions and specified exceptions are described in more detail under “Underwriters.”
All of these shares will, however, be able to be resold after the expiration of the lock-up periods described above, as well as pursuant to customary exceptions thereto or upon the waiver of the lock-up agreements by the representatives on behalf of the underwriters. We have registered shares of Class A common stock that we may issue under our equity compensation plans. Such shares can be freely sold in the public market upon issuance. As restrictions on resale end, the market price of our stock could decline if the holders of currently restricted shares of Class A common stock sell them or are perceived by the market as intending to sell them.
Following the lock-up periods described above, and assuming that the representatives, on behalf of the underwriters, do not release any parties from these agreements, all of the shares of our Class A common stock that are restricted securities or are held by our affiliates as of the date of this prospectus will be eligible for sale in the public market in compliance with Rule 144 under the Securities Act.
Registration Rights Agreement
We entered into a Registration Rights Agreement with MLSH 1 and MLSH 2 in connection with our IPO. The Registration Rights Agreement provides MLSH 1 and MLSH 2 certain registration rights whereby MLSH 1 and MLSH 2 can require us to register under the Securities Act shares of Class A common stock (including shares issuable to MLSH 1 upon exchange of its LLC Units). The Registration Rights Agreement also provides for piggyback registration rights for MLSH 1 and MLSH 2. See “Certain Relationships and Related Party Transactions—Registration Rights Agreement.”
53
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
MATERIAL U.S. FEDERAL INCOME TAX
CONSEQUENCES TO NON-U.S. HOLDERS
The following discussion is a summary of the material U.S. federal income tax consequences to Non-U.S. Holders (as defined below) of the ownership and disposition of our Class A common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax consequences. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or non-U.S. tax laws are not discussed. This discussion is based on the Code, Treasury regulations promulgated thereunder (the “Treasury Regulations”), judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service (the “IRS”), in each case as in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a Non-U.S. Holder of our common stock. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position to those discussed below regarding the tax consequences of the purchase, ownership and disposition of our Class A common stock.
This discussion is limited to Non-U.S. Holders that hold our Class A common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Non-U.S. Holder’s particular circumstances, including the impact of the Medicare tax on net investment income or the alternative minimum tax, or the consequences to persons subject to special tax accounting rules as a result of any item of gross income with respect to our Class A common stock being taken into account in an applicable financial statement. In addition, it does not address consequences relevant to Non-U.S. Holders subject to special rules, including, without limitation:
| • | U.S. expatriates and former citizens or long-term residents of the U.S.; |
| • | persons holding our common stock as part of a straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| • | banks, insurance companies and other financial institutions; |
| • | real estate investment trusts or regulated investment companies; |
| • | brokers, dealers or certain electing traders in securities that mark their securities positions to market for tax purposes; |
| • | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | tax-exempt organizations or governmental organizations; |
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| • | “qualified foreign pension funds” (within the meaning of Section 897(I)(2) of the Code and entities, all of the interests of which are held by qualified foreign pension funds); and |
| • | tax-qualified retirement plans. |
If any partnership or arrangement classified as a partnership for U.S. federal income tax purposes holds our Class A common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our Class A common stock and partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
54
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE OWNERSHIP AND DISPOSITION OF OUR CLASS A COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Definition of a Non-U.S. Holder
For purposes of this discussion, a “Non-U.S. Holder” is any beneficial owner of our Class A common stock that is neither a “United States person” nor an entity treated as a partnership for U.S. federal income tax purposes. A United States person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| • | an individual who is a citizen or resident of the U.S.; |
| • | a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized under the laws of the U.S. any state thereof, or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
Distributions
As described in the section entitled “Dividend Policy,” we do not anticipate declaring or paying dividends to holders of our Class A common stock in the foreseeable future. However, if we do make distributions of cash or property on our Class A common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will first constitute non-taxable returns of capital and be applied against and reduce a Non-U.S. Holder’s adjusted tax basis in its Class A common stock, but not below zero. Any excesses will be treated as capital gains and will be treated as described below under “Sale or Other Taxable Disposition.”
Subject to the discussion below on effectively connected income, backup withholding, and the Foreign Account Tax Compliance Act, dividends paid to a Non-U.S. Holder of our Class A common stock will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty, provided that the Non-U.S. Holder will be required to furnish to the applicable withholding agent prior to the payment of dividends a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate in order to avoid withholding with respect to such tax). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
If dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the U.S. to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the Non-U.S. Holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S.
55
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Any such effectively connected dividends will be subject to U.S. federal income tax on a net-income basis at the regular rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits (as adjusted for certain items), which will include such effectively connected dividends. Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Sale or Other Taxable Disposition
Subject to the discussion below on backup withholding and the Foreign Account Tax Compliance Act, a Non-U.S. Holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale or other taxable disposition of our Class A common stock unless:
| • | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the U.S. (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the U.S. to which such gain is attributable); |
| • | the Non-U.S. Holder is a nonresident alien individual present in the U.S. for 183 days or more during the taxable year of the disposition and certain other requirements are met; or |
| • | our common stock constitutes a U.S. real property interest (a “USRPI”), by reason of our status as a U.S. real property holding corporation (a “USRPHC”) for U.S. federal income tax purposes. |
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net-income basis at the regular rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits (as adjusted for certain items), which will include such effectively connected gain.
A Non-U.S. Holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on any gain derived from the disposition, which may generally be offset by U.S. source capital losses of the Non-U.S. Holder (even though the individual is not considered a resident of the U.S.), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we currently are not, and do not anticipate becoming, a USRPHC. Because the determination of whether we are a USRPHC depends, however, on the fair market value of our USRPIs relative to the fair market value of our non-U.S. real property interests and our other business assets, there can be no assurance we currently are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition by a Non-U.S. Holder of our common stock will not be subject to U.S. federal income tax if our Class A common stock is “regularly traded on an established securities market,” as defined by applicable Treasury Regulations, during the calendar year in which the disposition occurs, and such Non-U.S. Holder has owned, actually and constructively, five percent or less of our Class A common stock throughout the shorter of (1) the five-year period ending on the date of the sale or other taxable disposition and (2) the Non-U.S. Holder’s holding period. If we were to become a USRPHC and our Class A common stock were not considered to be “regularly traded on an established securities market” during the calendar year in which the relevant disposition by a Non-U.S. Holder occurred, such Non-U.S. Holder (regardless of the percentage of stock owned) would be subject to U.S. federal income tax on a sale or other taxable disposition of our Class A common stock and a 15% withholding tax would apply to the gross proceeds from such disposition.
Non-U.S. Holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
56
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Information Reporting and Backup Withholding
Payments of dividends on our Class A common stock generally will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know the Non-U.S. Holder is a United States person and the Non-U.S. Holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any dividends on our Class A common stock paid to the Non-U.S. Holder, regardless of whether any tax was actually withheld. In addition, proceeds of the sale or other taxable disposition of our common stock within the U.S. or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such Non-U.S. Holder is a United States person, or the Non-U.S. Holder otherwise establishes an exemption. If a Non-U.S. Holder does not provide the certification described above or the applicable withholding agent has actual knowledge or reason to know that such Non-U.S. Holder is a United States person, payments of dividends or of proceeds of the sale or other taxable disposition of our common stock may be subject to backup withholding at a rate currently equal to 24% of the gross proceeds of such dividend, sale, or taxable disposition. Proceeds of a disposition of our Class A common stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides or is established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts
Sections 1471 through 1474 of the Code and the Treasury Regulations and administrative guidance promulgated thereunder (commonly referred to as the “Foreign Account Tax Compliance Act” or “FATCA”) generally impose withholding at a rate of 30% in certain circumstances on dividends in respect of securities which are held by or through certain foreign financial institutions (including investment funds), unless any such institution (i) enters into, and complies with, an agreement with the IRS to report, on an annual basis, information with respect to interests in, and accounts maintained by, the institution that are owned by certain U.S. persons and by certain non-U.S. entities that are wholly or partially owned by U.S. persons and to withhold on certain payments, or (ii) if required under an intergovernmental agreement between the United States and an applicable foreign country, reports such information to its local tax authority, which will exchange such information with the U.S. authorities. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Accordingly, the entity through which shares of our Class A common stock are held will affect the determination of whether such withholding is required. Similarly, dividends in respect of our Class A common stock held by an investor that is a non-financial non-U.S. entity that does not qualify under certain exceptions will generally be subject to withholding at a rate of 30%, unless such entity either (i) certifies to the applicable withholding agent that such entity does not have any “substantial United States owners” or (ii) provides certain information regarding the entity’s “substantial United States owners,” which will in turn be provided to the U.S. Department of Treasury. Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our Class A common stock.
57
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Under the terms and subject to the conditions in an underwriting agreement dated the date of this prospectus, the underwriters named below, for whom Morgan Stanley & Co. LLC, and are acting as representatives, have severally agreed to purchase, and the selling stockholders have agreed to sell to them, severally, the number of shares of Class A common stock indicated below:
| Name | Number of Shares |
|||
| Morgan Stanley & Co. LLC |
||||
|
|
|
|||
| Total: |
||||
|
|
|
|||
The underwriters and the representatives are collectively referred to as the “underwriters” and the “representatives,” respectively. The underwriters are offering the shares of Class A common stock subject to their acceptance of the shares from the selling stockholders and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the shares of Class A common stock offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the shares of Class A common stock offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares of Class A common stock covered by the underwriters’ option to purchase additional shares described below.
The underwriters initially propose to offer part of the shares of Class A common stock directly to the public at the offering price listed on the cover page of this prospectus and part to certain dealers at a price that represents a concession not in excess of $ per share under the public offering price. After the initial offering of the shares of Class A common stock, the offering price and other selling terms may from time to time be varied by the representatives.
The selling stockholders have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to additional shares of Class A common stock at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to purchase about the same percentage of the additional shares of Class A common stock as the number listed next to the underwriter’s name in the preceding table bears to the total number of shares of Class A common stock listed next to the names of all underwriters in the preceding table.
The following table shows the per share and total public offering price, underwriting discounts and commissions, and proceeds before expenses to the selling stockholders. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
|
|
Total | |||||||||||
| Per Share |
No Exercise |
Full Exercise |
||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions to be paid by the selling stockholders |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to selling stockholders |
$ | $ | $ | |||||||||
The estimated offering expenses payable by us, exclusive of the underwriting discounts and commissions, are approximately $ . We have agreed to reimburse the underwriters for expense relating to clearance of this offering with FINRA up to $50,000.
58
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Our Class A common stock is listed on The Nasdaq Global Select Market under the trading symbol “MRVI.”
We, each of our directors and executive officers and the selling stockholders have agreed that, without the prior written consent of the representatives on behalf of the underwriters, we and they will not, and will not publicly disclose an intention to or cause any affiliate to, during the period ending 90 days after the date of this prospectus (the “restricted period”):
| • | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, including LLC Units (“Lock-Up Securities”); |
| • | file or confidentially submit any registration statement with the SEC relating to the offering of any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; or |
| • | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any Lock-Up Securities, |
whether any such transaction described above is to be settled by delivery of common stock or such other securities, in cash or otherwise. In addition, we and each such person agrees that, without the prior written consent of the representatives on behalf of the underwriters, we or such other person will not, during the restricted period, make any demand for, or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock.
The restrictions described in the immediately preceding paragraph do not apply to our directors, executive officers or the selling stockholders in certain circumstances, including:
| a. | transactions relating to shares of Class A common stock acquired in open market transactions after the completion of the offering of the shares, provided that no filing under Section 16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is required or voluntarily made in connection with subsequent sales of the Class A common stock or other securities acquired in such open market transactions; |
| b. | facilitating the establishment of a trading plan on behalf of a shareholder, officer or director of Maravai LifeSciences Holdings, Inc. pursuant to Rule 10b5-1 under the Exchange Act for the transfer of shares of Class A common stock, provided that such plan does not provide for the transfer of Class A common stock during the restricted period and provided further that to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made by Maravai LifeSciences Holdings, Inc. regarding the establishment of such plan, such announcement or filing shall include a statement to the effect that no transfer of Class A common stock may be made under such plan during the restricted period; |
| c. | transfers of Lock-Up Securities as a bona fide gift or gifts, to an immediate family member, to certain trusts or to a corporation, partnership, limited liability company, trust or other entity of which the holder and the immediate family of the holder are the legal and beneficial owner of all of the outstanding equity securities or similar interests, provided that each donee or transferee signs a lock-up agreement and provided further that certain filings under Section 16(a) of the Exchange Act or any other public filing or disclosure reporting a reduction in beneficial ownership of common stock shall not be required or voluntarily made during the restricted period; |
| d. | distributions of Lock-Up Securities to partners, members or stockholders, wholly-owned subsidiaries or affiliates of the holder, or if such transferee is not a natural person, to any direct or indirect partners, members or shareholders of such transferee until the Lock-Up Securities come to be held by a natural |
59
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| person, provided that certain transferees or distributees sign a lock-up agreement and provided further that any filing under Section 16(a) of the Exchange Act shall clearly indicate in the footnotes thereto the nature and conditions of such transfer, that such transfer is not for value and that the Lock-Up Securities subject to such transfer are subject to a lock-up agreement; |
| e. | transfers of Lock-Up Securities pursuant to a bona fide third party tender offer, merger, consolidation or other similar transaction or transactions made to all or substantially all holders of our common stock and approved by our board of directors, provided that in the event that such tender offer, merger, consolidation or other such transaction or transactions shall not be completed, the holder’s Lock-Up Securities shall remain subject to the provisions of the lock-up agreement during the restricted period; |
| f. | transfers of Lock-Up Securities as a result of the operation of law, pursuant to an order of a court or regulatory agency or by will, other testamentary document or intestate succession, provided that certain filings under Section 16(a) of the Exchange Act or any other public filing or disclosure reporting a reduction in beneficial ownership of common stock shall not be required or voluntarily made during the restricted period; |
| g. | repurchases of Lock-Up Securities pursuant to equity award agreements or other contractual arrangements providing for the right of such repurchase in connection with the termination of the holders employment or service with us, provided that no filing by the holder under the Exchange Act, or other public announcement, shall be voluntarily made in connection with any such transfer, and if the holder is required to file a report under the Exchange Act related thereto during the restricted period, such report shall disclose that such transfer was a result of our repurchase of the Lock-Up Securities pursuant to equity award agreements or other contractual arrangements in connection with the termination of the holders’s employment or service with us; |
| h. | receipt of Lock-Up Securities upon the exercise of an option to purchase Lock-Up Securities in connection with the termination or expiration of such option pursuant to its terms, provided that such option was granted pursuant to a stock option plan or other equity incentive plan described in this prospectus, provided further that any Lock-Up Securities received upon such exercise shall be subject to the lock-up agreement and provided further that no filing by the undersigned or any party (transferor or transferee) under the Exchange Act, or other public announcement shall be voluntarily made in connection with any such transfer, and if the holder is required to file a report under the Exchange Act related thereto during the restricted period, such report shall disclose that such transfer was a result of the exercise of options expiring or terminating in accordance with their terms and does not result in any aggregate reduction in the beneficial ownership; |
| i. | transfers of Lock-Up Securities to us pursuant to the exercise, on a “cashless” or “net exercise” basis, of any option to purchase Lock-Up Securities granted pursuant to stock option or equity incentive plans described in this prospectus, or for the purpose of satisfying any withholding taxes due as a result of the exercise of any option to purchase Lock-Up Securities or the vesting of any equity awards granted pursuant to stock option or equity incentive plans described in this prospectus, provided that no filing under the Exchange Act, or other public announcement, shall be voluntarily made in connection with any such transfer, and if the holder is required to file a report under the Exchange Act related thereto during the restricted period, such report shall disclose that such transfer was a result of such circumstances; |
| j. | in any exchange of LLC Units and a corresponding number of shares of our Class B common stock into or for shares of Class A common stock (or securities convertible into or exercisable or exchangeable for Class A common stock) in a manner consistent with the Exchange Agreement; provided that to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made regarding the exchange, such announcement or filing shall include a statement to the effect that such exchange occurred pursuant to the Exchange Agreement and provided further that no transfer of the shares of Class A common stock or other securities received upon exchange may be made during the restricted period; and |
60
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| k. | any pledge, hypothecation or other grant of a security interest in any Lock-Up Securities to one or more lending institutions as collateral or security for any loan, advance or extension of credit and the transfer of such Lock-Up Securities to such lending institution upon foreclosure of such Lock-Up Securities, provided that no subsequent transfer or sale of Lock-Up Securities by such lending institution shall be made during the restricted period. |
The restrictions also do not apply to us in certain circumstances, including:
| • | the sale of shares to the underwriters in this offering; |
| • | the issuance by us of shares of common stock upon the exercise of an option or a warrant or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing; |
| • | the sale or issuance of or entry into an agreement providing for the sale or issuance of common stock or securities convertible into, exercisable for or which are otherwise exchangeable for or represent the right to receive common stock in connection with the acquisition of securities, businesses, technology, property or other assets; joint ventures; commercial relationships or other strategic transactions, provided that the aggregate number of shares of common stock securities convertible into, exercisable for or which are otherwise exchangeable for or represent the right to receive common stock that we may sell or issue or agree to sell or issue pursuant to such circumstances shall not exceed % of the total number of shares of common stock outstanding immediately following the completion of the transactions contemplated by this prospectus to be completed as of that date, and provided further that all recipients of any such securities shall enter into a lock-up agreement for the remainder of the restricted period; |
| • | facilitating the establishment of a trading plan on behalf of a shareholder, officer or director of Maravai LifeSciences Holdings, Inc. pursuant to Rule 10b5-1 under the Exchange Act for the transfer of shares of Class A common stock, provided that such plan does not provide for the transfer of Class A common stock during the restricted period and provided further that to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made by us regarding the establishment of such plan, such announcement or filing shall include a statement to the effect that no transfer of Class A common stock may be made under such plan during the restricted period, and |
| • | the filing by us of a registration statement on Form S-8 relating to securities granted or to be granted pursuant to any compensation benefit plan described in this prospectus, and |
The representatives, in their discretion, may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time.
In addition, in connection with our IPO, all of our directors and executive officers and the holders of substantially all of our outstanding stock and stock options prior to our IPO agreed with the underwriters for our IPO that to and including May 18, 2021, without the prior written consent of the representatives of our IPO, they will be subject to lock-up restrictions substantially consistent with those described above, subject to the exceptions described in (a) through (j) above.
In order to facilitate the offering of the Class A common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the Class A common stock. Specifically, the underwriters may sell more shares than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for purchase by the underwriters under the option to purchase additional shares described above. The underwriters can close out a covered short sale by exercising the option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the underwriters will consider, among other things, the open market price of shares compared to the price available under the option to
61
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
purchase additional shares. The underwriters may also sell shares in excess of the option to purchase additional shares, creating a naked short position. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the Class A common stock in the open market after pricing that could adversely affect investors who purchase in this offering. As an additional means of facilitating this offering, the underwriters may bid for, and purchase, shares of Class A common stock in the open market to stabilize the price of the Class A common stock. These activities may raise or maintain the market price of the Class A common stock above independent market levels or prevent or retard a decline in the market price of the Class A common stock. The underwriters are not required to engage in these activities and may end any of these activities at any time.
We, the selling stockholders and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
A prospectus in electronic format may be made available on websites maintained by one or more underwriters, or selling group members, if any, participating in this offering. The representatives may agree to allocate a number of shares of Class A common stock to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters that may make Internet distributions on the same basis as other allocations.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for us, for which they received or will receive customary fees and expenses.
In addition, in the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities and instruments. Such investment and securities activities may involve our securities and instruments. The underwriters and their respective affiliates may also make investment recommendations or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long or short positions in such securities and instruments.
Selling Restrictions
European Economic Area
In relation to each Member State of the European Economic Area (each a “Member State”), no shares of our Class A common stock have been offered or will be offered pursuant to this offering to the public in that Member State prior to the publication of a prospectus in relation to shares of our Class A common stock which has been approved by the competent authority in that Member State or, where appropriate, approved in another Member State and notified to the competent authority in that Member State, all in accordance with the Prospectus Regulation, except that offers of shares of our Class A common stock may be made to the public in that Member State at any time under the following exemptions under the Prospectus Regulation:
| • | to any legal entity which is a qualified investor as defined in the Prospectus Regulation; |
| • | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior consent of the representatives for any such offer; or |
| • | In any other circumstances falling within Article 1(4) of the Prospectus Regulation; |
62
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
provided that no such offer of shares of our Class A common stock shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation, and each person who initially acquires any shares of our Class A common stock or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the representatives and us that it is a “qualified investor” as defined in the Prospectus Regulation.
In the case of any shares of our Class A common stock being offered to a financial intermediary as that term is used in Article 5 of the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares of our Class A common stock acquired by it in the offer have not been acquired on a nondiscretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares of our Class A common stock to the public other than their offer or resale in a Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares of our Class A common stock in any Member State means the communication in any form and by means of sufficient information on the terms of the offer and the shares of our Class A common stock to be offered so as to enable an investor to decide to purchase shares, the expression “Prospectus Regulation” means Regulation (EU) 2017/1129 (as amended).
This selling restriction is in addition to any other selling restrictions set out below.
United Kingdom
Each underwriter has represented and agreed that:
| (a) | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (“FSMA”)) received by it in connection with the issue or sale of the shares of Class A common stock in circumstances in which Section 21(1) of the FSMA does not apply to the Company; and |
| (b) | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of Class A common stock in, from or otherwise involving the United Kingdom. |
Canada
The Class A common stock may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the Class A common stock must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory of these rights or consult with a legal advisor.
63
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Hong Kong
The Class A common stock may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), (ii) to “professional investors” as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the Class A common stock may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares of Class A common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
Japan
The shares of Class A common stock have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended) (“FIEA”). The shares of Class A common stock may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the Class A common stock may not be circulated or distributed, nor may the Class A common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”)) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the shares of Class A common stock are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for 6 months after that corporation has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, (3) where no consideration is or will be given
64
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”).
Where the shares of Class A common stock are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for 6 months after that trust has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32.
Solely for the purposes of our obligations pursuant to Section 309B of the SFA, we have determined, and hereby notify all relevant persons (as defined in the Securities and Futures (Capital Markets Products) Regulations 2018 (“CMP Regulations”)) that the shares of Class common stock are “prescribed capital markets products” (as defined in the CMP Regulations) and Excluded Investment Products (as defined in MAS Notice SFA 04-N12: Notice on the Sale of Investment Products and MAS Notice FAA-N16: Notice on Recommendations on Investment Products).
Switzerland
This prospectus is not intended to constitute an offer or solicitation to purchase or invest in the Class A common stock. The Class A common stock may not be publicly offered, directly or indirectly, in Switzerland within the meaning of the Swiss Financial Services Act (“FinSA”) and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading venue (exchange or multilateral trading facility) in Switzerland. This document does not constitute a prospectus within the meaning of, and has been prepared without regard to, the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading venue (exchange or multilateral trading facility) in Switzerland. Neither this document nor any other offering or marketing material relating to the Class A common stock constitutes a prospectus pursuant to the FinSA, and neither this document nor any other offering or marketing material relating to the Class A common stock or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, or the Class A common stock have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of Class A common stock will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of Class A common stock has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of Class A common stock.
United Arab Emirates
The Class A common stock has not been, and are not being, publicly offered, sold, promoted or advertised in the United Arab Emirates (including the Dubai International Financial Centre) other than in compliance with the laws of the United Arab Emirates (and the Dubai International Financial Centre) governing the issue, offering and sale of securities. Further, this prospectus does not constitute a public offer of securities in the United Arab Emirates (including the Dubai International Financial Centre) and is not intended to be a public offer. This prospectus has not been approved by or filed with the Central Bank of the United Arab Emirates, the Securities and Commodities Authority or the Dubai Financial Services Authority.
65
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
The validity of the issuance of our Class A common stock offered in this prospectus will be passed upon for us by Kirkland & Ellis LLP, Chicago, Illinois. Certain legal matters will be passed upon for the underwriters by Davis Polk & Wardwell LLP, Menlo Park, California.
Ernst & Young LLP, independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2020, as set forth in their report which is incorporated by reference in this prospectus and elsewhere in the registration statement. Our consolidated financial statements are incorporated by reference in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act to register our Class A common stock being offered in this prospectus. This prospectus, which forms part of the registration statement, does not contain all of the information included in the registration statement and the attached exhibits. You will find additional information about us and our Class A common stock in the registration statement. References in this prospectus to any of our contracts, agreements or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contracts, agreements or documents.
The SEC maintains a website that contains reports, proxy statements and other information about companies like us, who file electronically with the SEC. The address of that website is http://www.sec.gov. This reference to the SEC’s website is an inactive textual reference only and is not a hyperlink.
We are subject to the reporting, proxy and information requirements of the Exchange Act, and are required to file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information are available on the website of the SEC referred to above, as well as on our website, https://www.maravai.com. This reference to our website is an inactive textual reference only and is not a hyperlink. The contents of, or other information accessible through, our website are not part of this prospectus. We furnish our stockholders with annual reports containing audited financial statements and quarterly reports containing unaudited interim financial statements for each of the first three quarters of each year.
66
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (File No. 001-39725):
| • | our Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on March 22, 2021; and |
| • | the description of our Class A common stock set forth in our registration statement on Form 8-A, filed with the SEC on November 19, 2020, including any amendments thereto or reports filed for the purposes of updating this description. |
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the SEC pursuant to the Exchange Act shall be incorporated by reference into this prospectus.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Maravai LifeSciences Holdings, Inc., 10770 Wateridge Circle Suite 200, Suite 200, San Diego, California 92121, Attention: Investor Relations.
You also may access these filings on our website at https://investors.maravai.com/. We do not incorporate the information on our website into this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
67
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Shares

MORGAN STANLEY
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than the underwriting discounts and commissions payable by us, in connection with the offer and sale of the securities being registered. All amounts shown are estimates except for the SEC registration fee and the FINRA filing fee.
| SEC registration fee |
$ | |||
| FINRA filing fee |
||||
| Printing expenses |
||||
| Legal fees and expenses |
||||
| Accounting fees and expenses |
||||
|
|
|
|||
| Miscellaneous expenses |
||||
|
|
|
|||
| Total expenses |
$ | |||
|
|
|
Item 14. Indemnification of Directors and Officers.
Section 102(b)(7) of the DGCL allows a corporation to provide in its certificate of incorporation that a director of the corporation will not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation provides for this limitation of liability.
Section 145 of the DGCL (“Section 145”) provides that a Delaware corporation may indemnify any person who was, is or is threatened to be made party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his conduct was illegal. A Delaware corporation may indemnify any persons who are, were or are threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit, provided that such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests, provided that no indemnification is permitted without judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him against the expenses which such officer or director has actually and reasonably incurred.
Section 145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise, against any liability asserted against him and incurred by him in such capacity, or arising out of his status as such, whether or not the corporation would otherwise have the power to indemnify him under Section 145.
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Our bylaws provide that we will indemnify our directors and officers to the fullest extent authorized by the DGCL and that we must also pay expenses incurred in defending any such proceeding in advance of its final disposition upon delivery of an undertaking by or on behalf of an indemnified person to repay all amounts so advanced if it should be determined ultimately that such person is not entitled to be indemnified under this section or otherwise.
We entered into indemnification agreements with each of our executive officers and directors. The indemnification agreements provide the executive officers and directors with contractual rights to indemnification, expense advancement and reimbursement, to the fullest extent permitted under the DGCL.
The indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, provision of our certificate of incorporation or bylaws, agreement, vote of stockholders or disinterested directors or otherwise.
We will maintain standard policies of insurance that provide coverage (1) to our directors and officers against loss arising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments that we may make to such directors and officers. The proposed form of Underwriting Agreement to be filed as Exhibit 1.1 to this Registration Statement provides for indemnification of our directors and officers by the underwriters party thereto against certain liabilities arising under the Securities Act of 1933 (the “Securities Act”) or otherwise.
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding securities sold by us within the past three years that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such securities and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
Since January 1, 2018, we have made sales of the following unregistered securities:
| • | On August 25, 2020, we issued 1,000 shares of Class A common stock to MLSH 1 for $0.01 per share in connection with our formation. |
| • | On November 19, 2020, we issued 28,965,664 shares of Class A common stock to MLSH 2 as partial consideration for the Blocker Mergers. |
| • | On November 19, 2020, we issued 168,654,981 shares of Class B common stock to MLSH 1 in exchange for LLC units in Topco LLC (the “LLC Units”). |
The issuances of the securities described above were exempt from registration under Section 4(a)(2) of the Securities Act as transactions by an issuer not involving any public offering.
Issuances under our 2020 Plan
| • | From November 24, 2020 to March 24, 2021, we granted to our directors, officers, employees, consultants and other service providers 131,235 options under our 2020 Plan. |
The offers and sales of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act, as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the above securities represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof. Appropriate legends were placed upon any stock certificates issued in these transactions. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Item 16. Exhibits and Financial Statement Schedules.
(i) Exhibits
| Exhibit Number |
Description | |
| 1.1* | Form of Underwriting Agreement. | |
| 3.1 | Amended and Restated Certificate of Incorporation of Maravai LifeSciences Holdings, Inc. dated November 19, 2020 (incorporated by reference to Exhibit 3.1 to Maravai Life Sciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 3.2 | Amended and Restated Bylaws of Maravai LifeSciences Holdings, Inc. dated November 19, 2020 (incorporated by reference to Exhibit 3.2 to Maravai Life Sciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 4.1 | Registration Rights Agreement, dated November 24, 2020, by and among Maravai LifeSciences Holdings, Inc. and the other signatories party thereto (incorporated by reference to Exhibit 4.1 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 5.1* | Opinion of Kirkland & Ellis LLP. | |
| 10.1+ | Maravai LifeSciences Holdings, Inc. 2020 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.1 to Maravai LifeSciences Holdings, Inc.’s Registration Statement on Form S-8 filed with the Securities and Exchange Commission on November 23, 2020). | |
| 10.2+§ | Senior Management Agreement, dated as of March 18, 2014, among Maravai Life Sciences Holdings, LLC, Maravai Life Sciences, Inc. and Carl Hull (incorporated by reference to Exhibit 10.2 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.3+§ | Amended and Restated Senior Management Agreement, dated as of August 4, 2015, among Maravai Life Sciences Holdings, LLC, Maravai Life Sciences, Inc. and Eric Tardif (incorporated by reference to Exhibit 10.3 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.4+§ | Senior Management Agreement, dated as of May 30, 2017, among Maravai Life Sciences Holdings, LLC, Maravai Life Sciences, Inc. and Kevin M. Herde (incorporated by reference to Exhibit 10.4 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.5+§ | Senior Management Agreement, dated as of December 27, 2017, among Maravai Life Sciences Holdings, LLC, TriLink Biotechnologies, LLC and Brian Neel (incorporated by reference to Exhibit 10.5 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.6+§ | Senior Management Agreement, dated as of December 27, 2017, among MLSC Holdings, LLC, Cygnus Technologies, LLC and Christine Dolan (incorporated by reference to Exhibit 10.6 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.7+ | Form of Stock Option Grant Notice and Stock Option Agreement (incorporated by reference to Exhibit 10.7 to Amendment No. 2 to Maravai LifeSciences Holdings, Inc.’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on November 13, 2020). | |
| 10.8 | Tax Receivable Agreement, dated as of November 19, 2020, by and among Maravai LifeSciences Holdings, Inc. and the other signatories party thereto (incorporated by reference to Exhibit 10.1 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 10.9 | Exchange Agreement, dated as of November 19, 2020, by and among Maravai LifeSciences Holdings, Inc. and the other signatories party thereto (incorporated by reference to Exhibit 10.2 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 10.10 | Second Amended and Restated Limited Liability Agreement of Maravai Topco Holdings, LLC, dated as of November 19, 2020, by and among Maravai LifeSciences Holdings, Inc. and the other signatories party thereto (incorporated by reference to Exhibit 10.3 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| Exhibit Number |
Description | |
| 10.11+ | Maravai LifeSciences Holdings, Inc. 2020 Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.2 to Maravai LifeSciences Holdings, Inc.’s Registration Statement on Form S-8 filed with the Securities and Exchange Commission on November 23, 2020). | |
| 10.12 | Form of Director and Officer Indemnification Agreement (incorporated by reference to Exhibit 10.12 to Maravai LifeSciences Holdings, Inc.’s Form S-1/A filed on November 9, 2020). | |
| 10.13§ | First Lien Credit Agreement, dated as of August 2, 2018, among Maravai Intermediate Holdings, LLC, Cygnus Technologies, LLC, Trilink Biotechnologies, LLC, Vector Laboratories, Inc., Maravai Topco Holdings, LLC and JPMorgan Chase Bank, N.A. (incorporated by reference to Exhibit 10.13 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.14§ | Second Lien Credit Agreement, dated as of August 2, 2018, among Maravai Intermediate Holdings, LLC, Cygnus Technologies, LLC, Trilink Biotechnologies, LLC, Vector Laboratories, Inc., Maravai Topco Holdings, LLC and Antares Capital LP (incorporated by reference to Exhibit 10.14 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.15§ | Distribution Agreement, dated January 14, 2019, between Cygnus Technologies, LLC and Beijing XMJ Scientific Co. Ltd. (incorporated by reference to Exhibit 10.15 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.16§ | Lease Agreement, dated as of January 10, 2020, between Shac Ingold Apartments LLC, and Vector Laboratories, Inc., as amended (incorporated by reference to Exhibit 10.16 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.17§ | Lease Agreement, dated as of September 23, 2019, between TransDulles Center, Inc., and Glen Research Corporation, as amended (incorporated by reference to Exhibit 10.17 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.18§ | Lease Agreement, dated as of July 13, 2018, between 10770 Wateridge Investors LLC, and Trilink Biotechnologies, LLC, as amended (incorporated by reference to Exhibit 10.18 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.19§ | Lease Agreement, dated as of October 6, 2016, between Arame, LLC, and Cygnus Technologies, LLC, as amended (incorporated by reference to Exhibit 10.19 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.20 | Second Amended and Restated Advisory Agreement, dated September 15, 2016, between GTCR Management XI LP, Vector Laboratories, Inc. and TriLink Biotechnologies, LLC (incorporated by reference to Exhibit 10.20 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.21+§ | Investment and Director Compensation Agreement, dated as of January 1, 2017, between Maravai Life Sciences Holdings, LLC, and Robert B. Hance (incorporated by reference to Exhibit 10.21 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.22+§ | Investment and Director Compensation Agreement, dated as of January 8, 2020, between Maravai Life Sciences Holdings, LLC, and Gregory T. Lucier (incorporated by reference to Exhibit 10.22 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.23+§ | Investment and Director Compensation Agreement, dated as of August 10, 2016, between Maravai Life Sciences Holdings, LLC, and Murali K. Prahalad (incorporated by reference to Exhibit 10.23 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
| 10.24§ | Credit Agreement, dated as of October 19, 2020, among Maravai Intermediate Holdings, LLC, Cygnus Technologies, LLC, Trilink Biotechnologies, LLC, Vector Laboratories, Inc., Maravai Topco Holdings, LLC and Morgan Stanley Senior Funding, Inc. (incorporated by reference to Exhibit 10.24 to Maravai LifeSciences Holdings, Inc.’s Form S-1 filed on October 29, 2020). | |
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| Exhibit Number |
Description | |
| 10.25 | Director Nomination Agreement, dated as of November 24, 2020, by and among Maravai LifeSciences Holdings, Inc. and the other signatories party thereto (incorporated by reference to Exhibit 10.5 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 10.26§‡ | Supply Agreement, dated as of October 9, 2020, by and among Pfizer Inc., BioNTech SE and TriLink BioTechnologies, LLC (incorporated by reference to Exhibit 10.26 to Maravai LifeSciences Holdings, Inc.’s Form S-1/A filed on November 9, 2020). | |
| 10.27+ | Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement (incorporated by reference to Exhibit 10.27 to Amendment No. 2 to Maravai LifeSciences Holdings, Inc.’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on November 13, 2020). | |
| 10.28+§ | Employment Agreement of Carl W. Hull, dated November 24, 2020, among Maravai LifeSciences Holdings, Inc., Maravai Intermediate Holdings, LLC and Carl W. Hull (incorporated by reference to Exhibit 10.8 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 10.29+§ | Employment Agreement of Kevin Herde, dated November 24, 2020, among Maravai LifeSciences Holdings, Inc., Maravai Intermediate Holdings, LLC and Kevin Herde (incorporated by reference to Exhibit 10.9 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 10.30+§ | Employment Agreement of Brian Neel, dated November 24, 2020, among Maravai LifeSciences Holdings, Inc., TriLink Biotechnologies, LLC and Brian Neel (incorporated by reference to Exhibit 10.10 to Maravai LifeSciences Holdings, Inc.’s Form 8-K filed on November 25, 2020). | |
| 21.1 | List of subsidiaries of Maravai LifeSciences Holdings, Inc. (incorporated by reference to Exhibit 21.1 to Maravai LifeSciences Holdings, Inc.’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 22, 2021). | |
| 23.1* | Consent of Independent Registered Public Accounting Firm. | |
| 23.2* | Consent of Kirkland & Ellis LLP (included in Exhibit 5.1). | |
| 24.1 | Powers of Attorney (included on signature page). | |
| 101.INS* | XBRL Instance Document. | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB* | XBRL Taxonomy Extension Labels Linkbase Document. | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. | |
| + | Indicates a management contract or compensatory plan or agreement. |
| * | To be filed by amendment. |
| § | Exhibits and schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K and will be provided on a supplemental basis to the Securities and Exchange Commission upon request. |
| ‡ | Certain portions of this document that constitute confidential information have been redacted in accordance with Regulation S-K, Item 601(b)(10). |
(ii) Financial statement schedules
No financial statement schedules are provided because the information called for is not applicable or is shown in the financial statements or notes.
Item 17. Undertakings.
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
Insofar as indemnification for liabilities under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in the form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective; and |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at the time shall be deemed to be the initial bona fide offering thereof. |
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on , 2021.
| MARAVAI LIFESCIENCES HOLDINGS, INC. | ||||
| By: |
| |||
| Name: | Carl W. Hull | |||
| Title: | Chief Executive Officer | |||
***
POWER OF ATTORNEY
The undersigned directors and officers of Maravai LifeSciences Holdings, Inc. hereby appoint each of Eric Tardif and Kevin Herde, as attorney-in-fact for the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, to sign and file with the Securities and Exchange Commission under the Securities Act of 1933 any and all amendments (including post-effective amendments) and exhibits to this registration statement on Form S-1 (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933) and any and all applications and other documents to be filed with the Securities and Exchange Commission pertaining to the registration of the securities covered hereby, with full power and authority to do and perform any and all acts and things whatsoever requisite and necessary or desirable, hereby ratifying and confirming all that said attorney-in-fact, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities indicated on , 2021.
| Signature |
Title | |
|
Carl W. Hull |
Chief Executive Officer and Director (Principal Executive Officer) | |
|
Kevin Herde |
Chief Financial Officer (Principal Financial Officer) | |
|
Sean Cunningham |
Director | |
|
Benjamin Daverman |
Director | |
|
Susannah Gray |
Director | |
|
Robert B. Hance |
Director | |
|
Jessica Hopfield |
Director | |
Confidential Treatment Requested by Maravai LifeSciences Holdings, Inc.
Pursuant to 17 C.F.R. Section 200.83
| Signature |
Title | |
|
Gregory T. Lucier |
Director | |
|
Luke Marker |
Director | |
|
Constantine Mihas |
Director | |
|
Murali K. Prahalad |
Director | |
|
Anat Ashkenazi |
Director | |